A Lewis för Is shown below, nowever, its formal charges are not minimized. Starting from this structure, complete the correct structure with minimized formal charges. S. Click to edit molecule
A Lewis för Is shown below, nowever, its formal charges are not minimized. Starting from this structure, complete the correct structure with minimized formal charges. S. Click to edit molecule
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:### Transcription for Educational Website
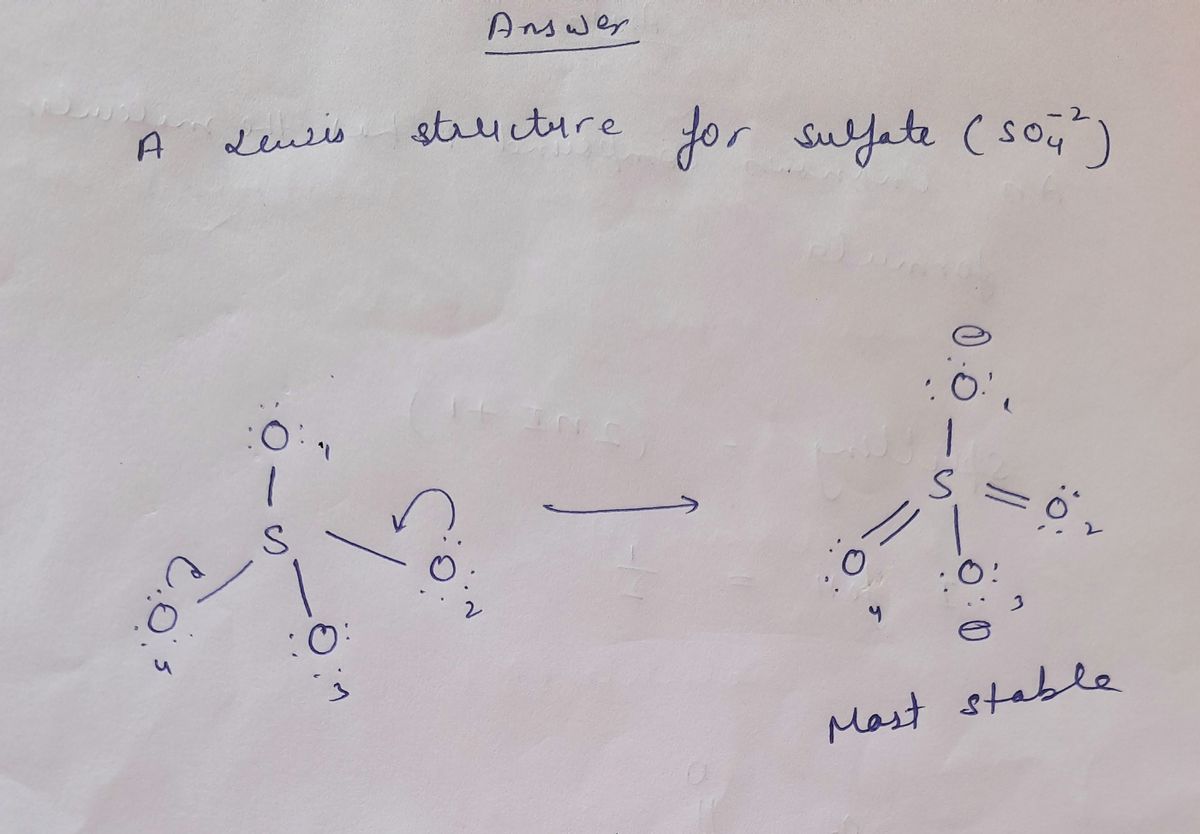
**Title: Understanding the Lewis Structure of Sulfate Ion (SO₄²⁻)**
**Introduction:**
A Lewis structure for sulfate (SO₄²⁻) is shown below, however, its formal charges are not minimized. Starting from this structure, complete the correct structure with minimized formal charges.
**Diagram Explained:**
The diagram shows the initial Lewis structure for the sulfate ion (SO₄²⁻). In this structure:
- The central atom is sulfur (S).
- Four oxygen atoms (O) are bonded to the sulfur.
- Each oxygen atom has six valence electrons represented as dots around them.
- Lines between sulfur and oxygen atoms represent single covalent bonds.
- Note that the current arrangement does not minimize the formal charges on the atoms.
Your task is to adjust the electron pairs and bonds to achieve minimized formal charges for the entire molecule.
**Interactive Element:**
Click to edit molecule - Engage with the molecule by adjusting the placement of electrons and modifying bonds to better understand the chemical structure and stability of the sulfate ion.
---
This layout encourages learners to interact with the diagram to explore how formal charges can be minimized through proper arrangement of electrons and bonds in the sulfate ion.
Expert Solution
Step 1⁰
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY