4⁰K is an unusual isotope, in that it decays by negative beta emission, positive beta emission, and electron capture. Find the Q values for: (b) positive beta emission
Q: An old wooden tool, containing 120.0 grams of carbon, is found in an ancient tomb. The tool emits…
A: Given, mass of carbon is m=120g emitting rate of electrons from the beta decay of C614 is…
Q: (Answer in MeV) Question in image
A: The expression for the energy absorbed is,
Q: Atts, you have a pure sample of an unknown substance. At t= 293 s, 36.8% of the substance remains.…
A: Radioactive substance remaining at t=293s, N=36.8100×N0=0.368N0
Q: 3. An ancient club is found that contains 150 g of pure carbon and has an activity of 5.5 decays per…
A:
Q: Gamma rays may be used to kill pathogens in ground beef. One irradiation facility uses a 60C source…
A: Given: The activity of gamma rays is A=1×106 The energy of first gamma ray is E1=1.17 MeV. The…
Q: A living specimen in equilibrium with the atmosphere contains one atom of 14C (half-life = 5730 yr)…
A: No. of atoms of any sample remaining after a time t is given as: N(t)=N0e-tλ The solution of this…
Q: The radionuclide 1311 Has a half-life of 100 hours. The activity of a m = 60mg of 1311 Is about: (N.…
A: Given: half life T1/2= 100 hours= 100*3600= 360000 sec we know that T1/2 = 0.693/λ λ is decay…
Q: As part of a radiotherapy course a patient is injected with a sodium phosphate solution containing…
A: Given: The mass of the phosphorus 32 is MP = 31.973908 u The mass of the product atom is MS =…
Q: The stable isotope of sodium is 23Na. (a) Find the total binding energy and the binding energy per…
A:
Q: Samarium-153, a radioactive isotope with a half-life of approximately 48 hours, is used to treat…
A: Given, Half life, T1/2=48hours=2 days
Q: Question 10 of 22 Radium (A = 226, Z=88) decays to Radon (A = 222, Z=86) with a half-life of 1600…
A: in this question we discuss about decay of radioactive substances.
Q: A living specimen in equilibrium with the atmosphere contains one atom of 14C (half-life = 5 730 yr)…
A: Determine the number of carbon molecules per mole. Determine the initial number of atoms of C-14.
Q: A radioactive sample of 60Co (t₁/2 = 5.271 y) has a ß- activity of 4.4 X 107 Bq. How many grams of…
A: When the number of protons and neutrons inside a nucleus is not balanced some nuclei will become…
Q: Two radioactive waste products from nuclear reactors are strontium Sr (712= 29.1yr) and cesium Cs…
A:
Q: A 70-kg worker is accidentally exposed for 2.0 minutes to a 21-mCi source of beta radiation from a…
A: (a) Let M, T, and R denote the person’s mass, the time for exposure, and the radioactive sample’s…
Q: A patient with prostate cancer is treated with implanted seeds that contain the samarium isotope…
A: Given Data:Half life Energy Activity Mass To Determine:The equivalent dose that the gland receives…
Q: The isotope 145Pr decays by emission of beta particles with an energy of 1.80MeV each. Suppose a…
A: Formula, D = A x E x t / M where, D is the absorbed dose in gray (Gy) or rad A is…
Q: In the 1986 disaster at the Chernobyl reactor in eastern Europe, about 1 8 of the 137 Cs present in…
A: It is a measure of radiation dose to tissue. It is less fundamental than absorbed dose, but has…
Q: Iodine-131, a beta emitter, has a half-life of 8 days. A 2-gram sample of initially pure iodine-131…
A:
Q: A living specimen in equilibrium with the atmosphere contains one atom of 14C (half- life = 5 730…
A: In this question we have to find the age of the given sample in the nearest year. Please give…
Q: A piece of charcoal used for cooking is found at the remains of an ancient campsite. A 0.92-kg…
A: The initial activity of the sample can be obtained as R0=0.92×103 g15.0 decays/min11g=1.38×104/min
Q: A 60-kg person accidentally ingests a small source of alpha particles (RBE=15). The activity of the…
A: (i) Number of particles absorbed is equal to the number of particles decayed
Q: (d) An a particle is confined in a 'Dy nucleus. Assuming the speed of the a particle is…
A: Uncertainty principle states that it is impossible to measure distance and momentum of a particle…
Q: A living specimen in equilibrium with the atmosphere contains one atom of ¹4℃ (half-life = 5 730 yr)…
A:
Q: The reaction shown below has a Q value of 13.574 MeV. H+ N - C+ He Determine the atomic mass (in u)…
A: The Q value of the given nuclear reaction is given by, Q=sum of mass of reactants -sum of mass of…
Q: b) Complete the following decay reaction by identifying both the temporary nucleus (??) and the end…
A:

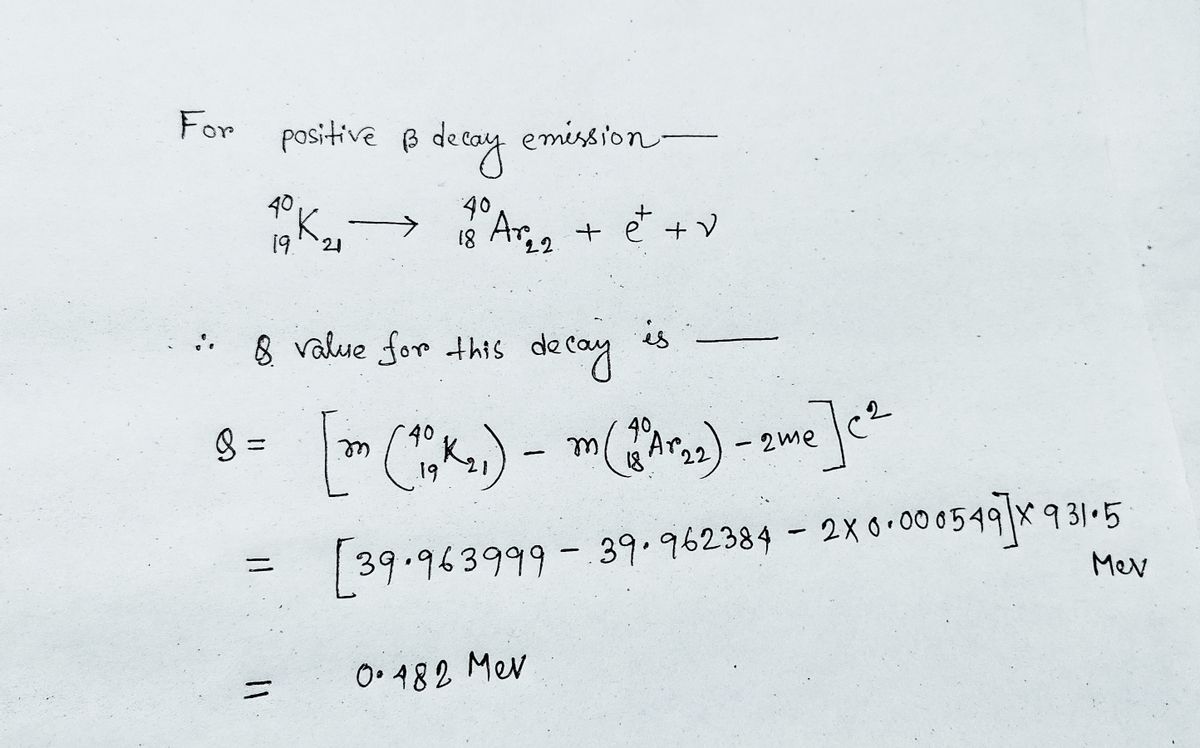
Step by step
Solved in 2 steps with 2 images

- You have a sample of unknown medication that is radioactive. You believe you should be able to identify the medication if you can deduce its half-life. You measure its initial activity as 2.30e+06 Bq. 10.4 hours later you measure its activity as 8.10e+05 Bq. a) What is the half-life of this unknown sample? hour b) How many nuclides are present when the activity is 8.10e+05 Bq?By READING the N vs t graph shown below, determine No & the half-life. N (x10¹ atoms) 120 90. 60. 30. 1 2 3 4 5 6 7 8 9 10 t(y) No = half-life = atomsQuestion 3. Two nuclei having the same mass number are known as isobars. (a)Calculate the difference in binding energy per nucleon for the following two isobars Na and Mg. (b) How do you account for this difference? (The mass of ?3Mg = 22.994 127 u.) 23
- Part A Calculate (in MeV) the total binding energy for 40 Ar. Express your answer in mega-electron volts to four significant figures. ? MeV Submit Previous Answers Request Answer X Incorrect; Try Again; 7 attempts remaining Part B Calculate (in MeV) the binding energy per nucleon for 40 Ar. Express your answer in mega-electron volts to three significant figures. Ην ΑΣφ ? Var = MeV Submit Request Answer Part C Calculate (in MeV) the total binding energy for 40K. Express your answer in mega-electron volts to four significant figures. nνα ΑΣφ ? BK = MeV Submit Previous Answers Request AnswerI need the answer as soon as possible