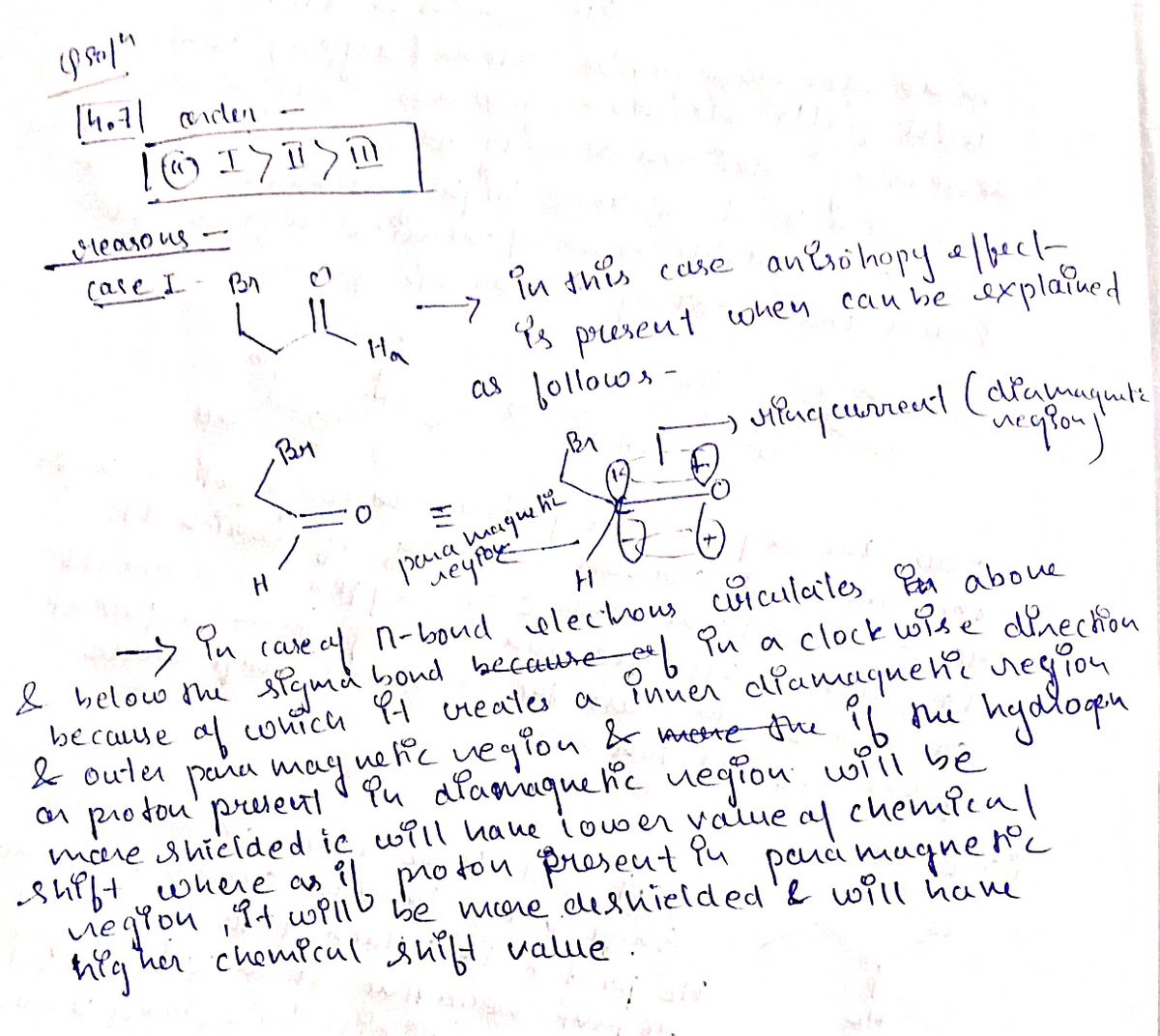
4.7. Rank the following protons or sets of protons according to their expected 1H NMR chemical shifts from highest shift (most deshielded) to lowest shift (most shielded): II III a. I > || > II b. I> III > || c. III >I> || d. Il >|> II e. Il > III > I 4.8. The compound [14]-annulene is highly reactive and shows only two 1H NMR signals – one at ~0 ppm and the other -7.6 ppm. Using benzene as a model for diamagnetic anisotropy, which of the following is expected to be true for the 'H signals of {14}-annulene? a. The blue are 7.6 ppm and the red are 0 ppm b. The red are 7.6 ppm and the blue are 0 ppm c. Some of the red and some of the blue give rise to each signal d. More information is needed
Electronic Effects
The effect of electrons that are located in the chemical bonds within the atoms of the molecule is termed an electronic effect. The electronic effect is also explained as the effect through which the reactivity of the compound in one portion is controlled by the electron repulsion or attraction producing in another portion of the molecule.
Drawing Resonance Forms
In organic chemistry, resonance may be a mental exercise that illustrates the delocalization of electrons inside molecules within the valence bond theory of octet bonding. It entails creating several Lewis structures that, when combined, reflect the molecule's entire electronic structure. One Lewis diagram cannot explain the bonding (lone pair, double bond, octet) elaborately. A hybrid describes a combination of possible resonance structures that represents the entire delocalization of electrons within the molecule.
Using Molecular Structure To Predict Equilibrium
Equilibrium does not always imply an equal presence of reactants and products. This signifies that the reaction reaches a point when reactant and product quantities remain constant as the rate of forward and backward reaction is the same. Molecular structures of various compounds can help in predicting equilibrium.
I don't know how or understand either of these questions. Please explain possibly, or show me with your work to better understand the answers to these questions!
![4.7. Rank the following protons or sets of protons according to their expected 'H NMR chemical shifts from
highest shift (most deshielded) to lowest shift (most shielded):
H.
II
III
a. I > || > II
b. I > III > ||
c. III > |> ||
d. Il >|> II
e. Il > III > |
4.8. The compound [14]-annulene is highly reactive and shows only two
1H NMR signals – one at ~0 ppm and the other ~7.6 ppm.
Using benzene as a model for diamagnetic anisotropy, which of the following
is expected to be true for the 'H signals of (14}-annulene?
HH
нн
a. The blue are 7.6 ppm and the red are 0 ppm
b. The red are 7.6 ppm and the blue are 0 ppm
c. Some of the red and some of the blue give rise to each signal
d. More information is needed](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F96a24c9f-fdcb-4c87-93bd-14e420e28946%2F427abc6d-934a-462c-b90f-70b84983d306%2Fojw6nfk_processed.jpeg&w=3840&q=75)
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images









