4. (a) Determine the reduced pressure (P,), volume (V,), and temperature (T,) for a 50 L sample of water at 23 bar and 400 K. For H20 gas, T. = 647.14 K, Pc = 220.64 bar, and Ve =55.95 L. (b) Determine the pressure, volume and temperature for a sample of hydrogen which is in a corresponding state with the H2O in part (a). For hydrogen, Te = 32.98 K, Pc = 12.93 bar and Vmc =64.20 L.
4. (a) Determine the reduced pressure (P,), volume (V,), and temperature (T,) for a 50 L sample of water at 23 bar and 400 K. For H20 gas, T. = 647.14 K, Pc = 220.64 bar, and Ve =55.95 L. (b) Determine the pressure, volume and temperature for a sample of hydrogen which is in a corresponding state with the H2O in part (a). For hydrogen, Te = 32.98 K, Pc = 12.93 bar and Vmc =64.20 L.
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.4QAP
Related questions
Question
Vc=60.20L

Transcribed Image Text:| 4. (a) Determine the reduced pressure (P,), volume (V,), and temperature (T, ) for a 50 L sample of water
at 23 bar and 400 K. For H20 gas, Te = 647.14 K, Pc = 220.64 bar, and Ve =55.95 L.
| (b) Determine the pressure, volume and temperature for a sample of hydrogen which is in a corresponding
state with the H2O in part (a). For hydrogen, Te = 32.98 K, Pc = 12.93 bar and Vm,c =64.20 L.
Expert Solution
Step 1 Given
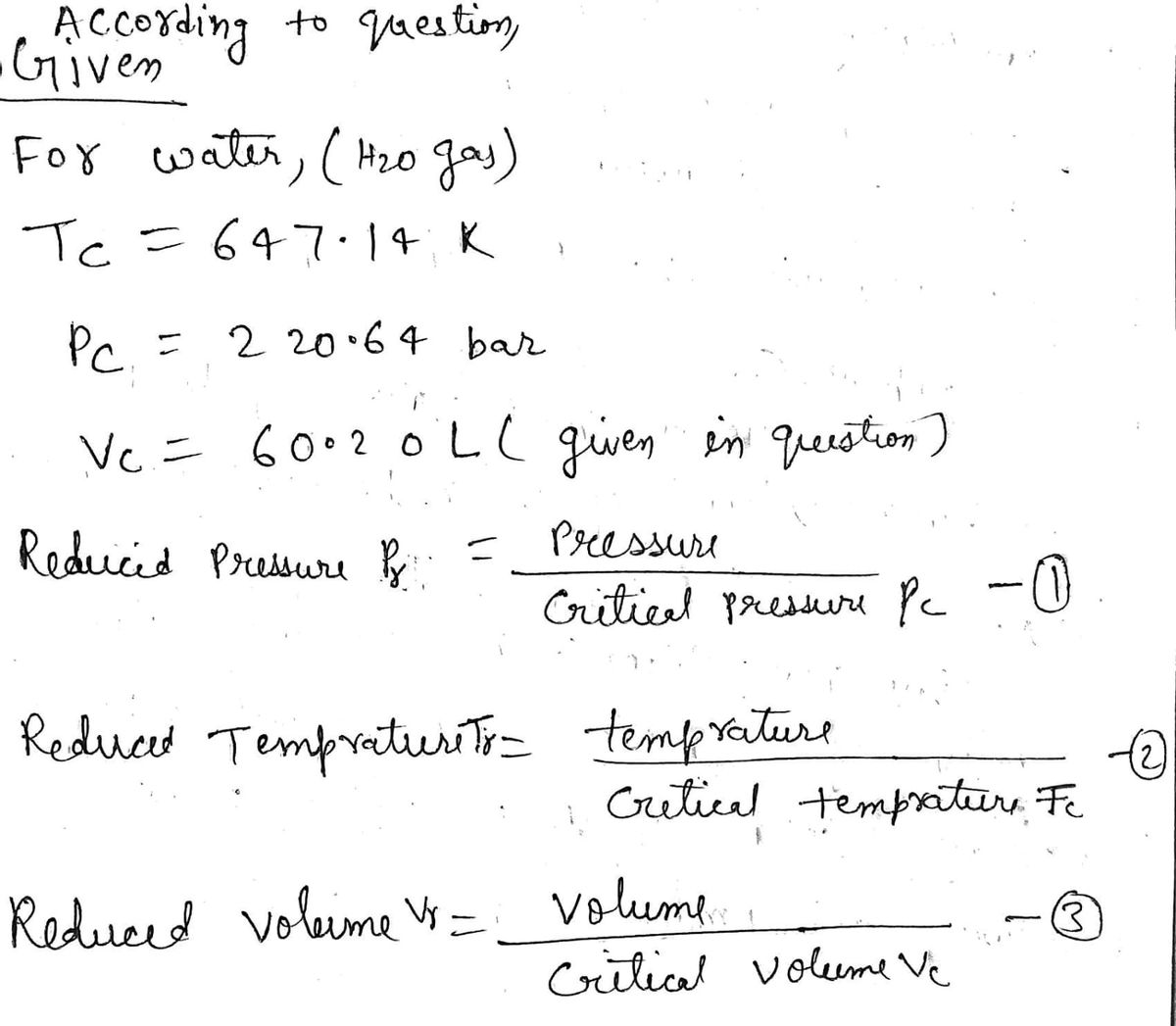
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning