1. Determine the ratio of diffusion coefficients for the following thre gnificance. Useful diffusion data can be found in Appendix B of the a) Carbon diffusing in a-Fe at 500°C and 700°C. b) Carbon and manganese diffusing in a-Fe at 800°C. c) Carbon diffusing in y-Fe and a-Fe at 800°C.
1. Determine the ratio of diffusion coefficients for the following thre gnificance. Useful diffusion data can be found in Appendix B of the a) Carbon diffusing in a-Fe at 500°C and 700°C. b) Carbon and manganese diffusing in a-Fe at 800°C. c) Carbon diffusing in y-Fe and a-Fe at 800°C.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:Q1. Determine the ratio of diffusion coefficients for the following three examples and explain their
significance. Useful diffusion data can be found in Appendix B of the textbook.
a) Carbon diffusing in a-Fe at 500°C and 700°C.
b) Carbon and manganese diffusing in a-Fe at 800°C.
c) Carbon diffusing in y-Fe and a-Fe at 800°C.

Transcribed Image Text:B.1
3.2
Appendix B
Selected diffusion data
Self-diffusivity of the elements
Matrix
Ag
Al
Cr
Cu
a-Fe
7-Fe
Ni
Si
D=Do exp(-Q/RT), R = 8.314J/mol K
A1₂03
Cr₂03
Coo
Fe₂03
MgO
NiO
Ni,Al
Do (m²/s)
1.06 x 10-4
1.71 x 10-4
2.00 × 10-5
2.32 x 10-4
2.01 x 10-4
2.20 × 10-5
X
2.22 × 10-4
0.9
242
Solute diffusivity in compounds
Q(kJ/mol)
Matrix Diffusing species Do (m²/s)
2.80 × 10-3
0.4
2.15 x 10-7
1.30 × 10²
2.49 × 10-5
4.80 × 10-8
1.90 x 10-6
186
142
Al
Cr
Co
Fe
Mg
Ni
Ni
308
206
240
268
289
495
Q(kJ/mol)
477
418
144
419
330
202
284
Comments
nod
Solute diffusivity in alloys
Matrix
Al
Cu
a-Fe
Nb
Ni
Diffusing species
Si
Ta
W
JNOů u od ≤ ≤ z ZBUJO
Zn
Zr
7-Fe
(0,00M
Fesnid-of
Ni
Si
Zn
Η
Ni
Sb
Zn
C
hate M J N
Ni
Cr
H
Mn
Cr
Ni
H
Au
C
Cr
Cu
Femei
H
Do (m²/s)
1.50 x 10-5
4.10 × 10-13
2.90 × 10-12
9.0 × 10-5
W
Sb
H
Mo
N
Cu
H
1.40 × 10-4
1.93 x 10-4
1.96 × 10-7
1.93 x 10-4
3.40 × 10-5
3.40 × 10-5
2.20 × 10-4
2.53 x 10-4
4.70 x 10-5
2.30 × 10-⁹
4.9 × 10-5
8 x 10-7
9.90 × 10-4
6.90 × 10-3
1.50 × 10-5
2.2 × 10-5
3.00 × 10-4
7.70 x 10-5
5 × 10-10
2.75 x 10-7
1.20 × 10-5
1.10 × 10-4
X
5.70 × 10-5
8.0 x 10-5
6.9 × 10-⁹
4.8 × 10-⁹
X
Q(kJ/mol) Comments
126
58
66
176
129
226
2.0 × 10
1.3 x 10-3
4.4 x 10-10
3.70 x 10-7
5.4 x 10-4
2.00 × 10-4
7.7 x 10-6
28.8
232
128
191
122
240
244
6.6
276
76
259
265
142
268
255
280
10.2
196
142
272
258
255
40.3
39.3
299
383
13.
13.5
460
260
125
453
(T < 631 K)
(T> 631 K)
LE
Expert Solution
Step 1: The Diffusion equation
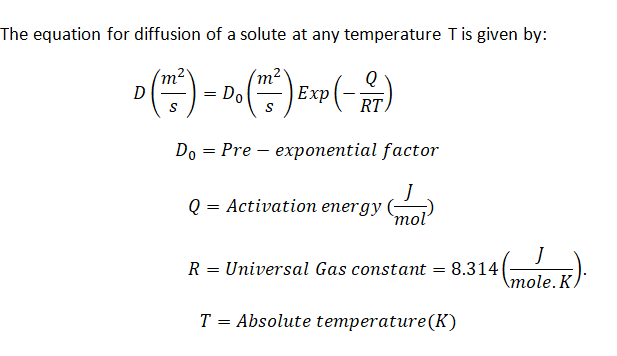
Step by step
Solved in 5 steps with 6 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The