
Concept explainers
Interpretation:
The mechanism for each of the given reactions are to be written.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron-rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 The reaction in which there is addition of halogen (F, Cl, Br, and I) to the compound is called halogenation reaction.
舧 A type of halogenation in which
舧 Hydride shift is defined as the rearrangement of hydrogen atoms.
Answer to Problem 1P
Solution:
(a)
(b)
(c)

(d)

Explanation of Solution
a)
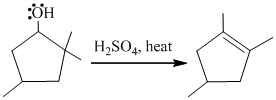
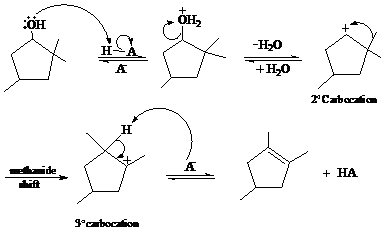
In the given reaction, cyclopentanol reacts with sulfuric acid in presence of heat to form the required product. The mechanism of this reaction takes place when the hydroxy group, which is having lone pair of electron, attacks on hydrogen atom (H) and withdraw it. After that equilibrium forms and removal of water takes place, which leads to the formation of secondary carbocation. The secondary carbocations are very unstable so methanide shift takes place and stable tertiary carbocation forms.
The mechanism of the reaction is as follows:
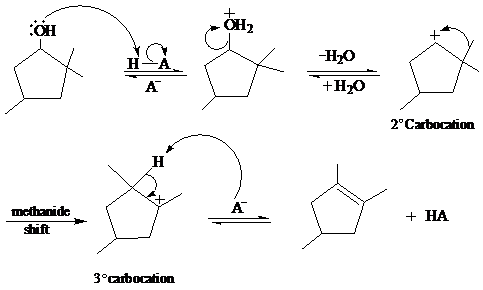
b)
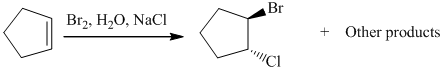
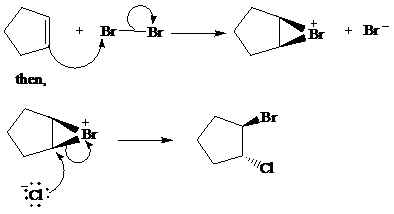
The given reaction take place when cyclopentene ring reacts with bromine in presence of light by the help of free radical halogenation. In the mechanism, the cyclopentene attacks on Br and addition of Br cation takes place at cyclopentene ring. The cyclopentene ring becomes electron deficient after the addition of Br so addition of chlorine atom takes place at cyclopentene ring and this leads to the formation of required product.
The mechanism is as follows:
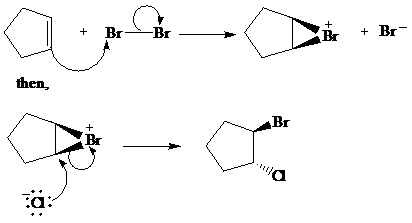
c) The other products from the reaction given in part (b).
From the above reaction, one more product is formed. When addition of chlorine atom takes place at electron deficient cyclopentene ring then addition of chlorine takes place but the product formed here is an enantiomer of the product formed above. So two products are formed, both are in equimolar amount and are enantiomer of each other.
The second product of the reaction is as follows:

d)
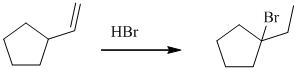
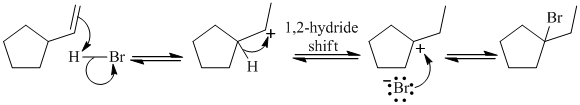
The given reaction takes place, when
The mechanism is represented below:
Want to see more full solutions like this?
Chapter FRP Solutions
EBK ORGANIC CHEMISTRY
- If a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forwardI have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forward
- no Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forwardno Ai walkthroughsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





