
Concept explainers
(a)
Interpretation:
For the given molecule, the complete IUPAC name is to be written.
Concept introduction:
To write the IUPAC name, first, it is important to determine the highest-priority functional group present that requires a suffix referring to Table E-1. For the molecules having two or more highest-priority
The main chain or ring containing the highest-priority functional group is to be determined. The next step is to number the main chain or ring such that carbon atoms involving highest-priority functional group receive the lowest possible numbers. The locator number for the highest-priority functional group is written immediately before the suffix unless needed. All other functional groups in the molecule are treated as substituents and appear in the name as a prefix. Prefixes such as ‘di’, ‘tri’, ‘tetra’... etc. are used to indicate the number of identical substituents attached. The substituents are named in alphabetical order.
In the IUPAC name of the molecule, the stereochemical configurations at the chiral centers are included as R/S. When assigning priorities to substituents, the atom having the greater
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then, the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined, and that arrangement is reversed before assigning R or S.
When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem E.32P
The complete IUPAC name for the given molecule, considering the given stereochemistry, is
Explanation of Solution
The given molecule is:
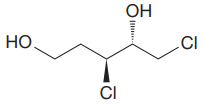
The molecule above contains two
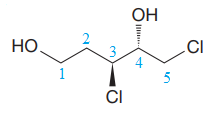
The corresponding locator numbers are also added before the prefix ‘di’. Thus, the name of the root is
There are two chiral centers in the given molecule at C3 and C4 carbon atoms.
The C3 carbon attached to Chlorine atom by a wedge bond has the S configuration, while the C4 carbon attached to the
The complete IUPAC name for the given molecule considering the given stereochemistry
is written above.
(b)
Interpretation:
For the given molecule, the complete IUPAC name is to be written.
Concept introduction:
To write the IUPAC name, first, it is important to determine the highest-priority functional group present that requires a suffix referring to Table E-1. For the molecules having two or more highest-priority functional groups, the rules are slightly different. In the IUPAC name of such molecules, the final ‘e’ of ‘ane’, ‘ene’, or ‘yne’ is not removed in the suffix. Prefixes such as ‘di’, ‘tri, etc are written immediately before the suffix to specify the number of highest-priority functional groups. Add a locator number for each of the highest-priority functional groups immediately before the prefixes.
The main chain or ring containing the highest-priority functional group is to be determined. The next step is to number the main chain or ring such that carbon atoms involving the highest-priority functional group receive the lowest possible numbers. The locator number for the highest-priority functional group is written immediately before the suffix unless needed. All other functional groups in the molecule are treated as substituents and appear in the name as a prefix. Prefixes such as ‘di’, ‘tri’, ‘tetra’… etc. are used to indicate the number of identical substituents attached. The substituents are named in alphabetical order.
In the IUPAC name of the molecule, the stereochemical configurations at the chiral centers are included as R/S. When assigning priorities to substituents, the atom having the greater atomic number has higher priority.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then, the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom, and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem E.32P
The complete IUPAC name for the given molecule is
Explanation of Solution
The given molecule is:
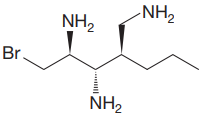
The molecule above contains three
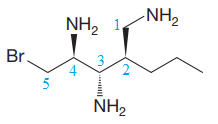
The corresponding locator numbers are also added before the prefix ‘tri’. Thus, the name of the root is
The carbon atoms C2 and C3 have two substituents attached.
One propyl group is attached to the C2 carbon atom of the root, while one bromine atom is attached to the C5 carbon atom of the root. The substituents are named in alphabetical order.
Thus, the complete IUPAC name for the compound without considering the stereochemistry is
There are three chiral centers in the given molecule.
The C2 carbon atom has R configuration.
The C3 carbon attached to
The C4 carbon attached to
Thus, the IUPAC name of the compound, considering the given stereochemistry is,
The complete IUPAC name for the given molecule considering the given stereochemistry
is written above.
(c)
Interpretation:
For the given molecule, the complete IUPAC name is to be written.
Concept introduction:
To write the IUPAC name, first, it is important to determine the highest-priority functional group present that requires a suffix referring to Table E-1. For the molecules having two or more highest-priority functional groups, the rules are slightly different. In the IUPAC name of such molecules, the final ‘e’ of ‘ane’, ‘ene’, or ‘yne’ is not removed in the suffix. Prefixes such as ‘di’, ‘tri, etc are written immediately before the suffix to specify the number of highest-priority functional groups. Add a locator number for each of the highest-priority functional groups immediately before the prefixes.
The main chain or ring is to be determined to be the one that contains the highest-priority functional groups. The next step is to number the main chain or ring such that carbon atoms involving highest-priority functional group receives the lowest possible numbers. The locator number for the highest-priority functional group is written immediately before the suffix unless needed. All other functional groups in the molecule are treated as substituents and appear in the name as a prefix. Prefixes such as ‘di’, ‘tri’, ‘tetra’… etc. are used to indicate the number of identical substituents attached. The substituents are named in alphabetical order.
In the IUPAC name of the molecule, the stereochemical configurations at the chiral centers are included as R/S. When assigning priorities to substituents, the atom having the greater atomic number has higher priority.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged clockwise, the configuration is R.
When the fourth priority substituent is pointing away (it is attached by a dash bond) and the first, second, and third priority substituents are arranged counterclockwise, the configuration is S.
If the fourth priority substituent is attached by a wedge bond, then the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
If the fourth priority substituent is in the plane of the page, then it is switched with the substituent that points away. Then, the clockwise or counterclockwise arrangement of the first, second, and third priority substituents is determined and that arrangement is reversed before assigning R or S.
When writing the IUPAC name, the R or S designation is written in parenthesis for each asymmetric carbon atom and hyphens are used to separate those designations from the rest of the IUPAC name.
Answer to Problem E.32P
The complete IUPAC name for the given molecule is
Explanation of Solution
The given molecule is:
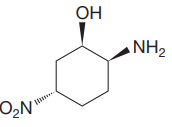
The molecule above contains one
The numbering is shown below:
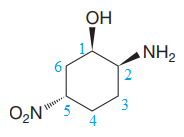
The name of the root is
There is one
Thus, the complete IUPAC name without considering the stereochemistry for the compound is
There are three chiral centers in the molecule.
The C1 carbon atom attached to the
The C2 carbon attached to the
The C5 carbon attached to the
Thus, the IUPAC name of the compound considering the given stereochemistry is
The complete IUPAC name for the given molecule considering the given stereochemistry
is written above.
Want to see more full solutions like this?
Chapter E Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
- What is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forward
- Predict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forward
- Predict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forward
