
Concept explainers
(a)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
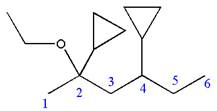
Explanation of Solution
The given IUPAC name is
The root name ‘hexane’ indicates the parent chain is of six carbon atoms.
Thus, the chain is numbered as shown below:
![]()
In the IUPAC name
Hence, the structure of
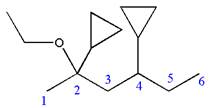
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(b)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for
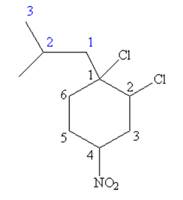
Explanation of Solution
The given IUPAC name is
The root name cyclohexane indicates the parent is a ring of six carbon atoms.
Hence, the structure of
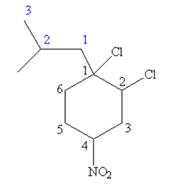
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(c)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
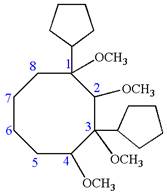
Explanation of Solution
The given IUPAC name is
The root name cyclooctane indicates the parent is a ring of eight carbon atoms.
Hence, the structure of
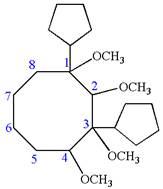
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(d)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
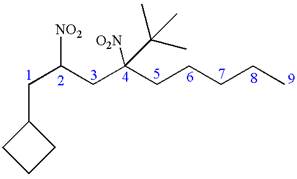
Explanation of Solution
The given IUPAC name is
The root name nonane indicates the parent is a chain of nine carbon atoms.
![]()
Hence, the structure of
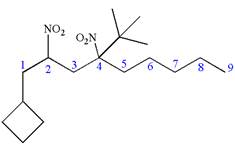
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(e)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
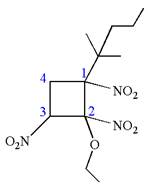
Explanation of Solution
The given IUPAC name is
The root name cyclobutane indicates the parent is a ring of four carbon atoms.
Hence, the structure of
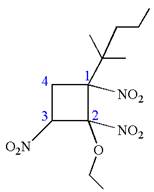
The structure for the given molecules is drawn from the root name, prefix and locator of substituents.
(f)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
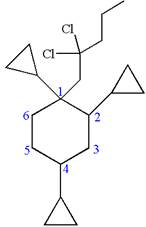
Explanation of Solution
The given IUPAC name is
The root name cyclohexane indicates the parent is a ring of six carbon atoms.
Hence, the structure of
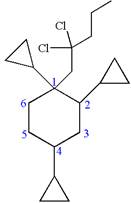
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(g)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
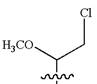
The structure for the given IUPAC name is as follows:
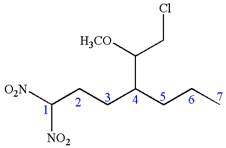
Explanation of Solution
The given IUPAC name is
The root name heptane indicates the parent is a chain of seven carbon atoms.
![]()
Hence, the structure of
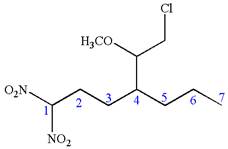
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
(h)
Interpretation:
The structure for the given IUPAC name is to be drawn.
Concept introduction:
The root name in the IUPAC name is the parent alkane. The longest continuous carbon chain must be drawn from the root name and numbered. The substituents are attached to the desired carbon number according to locator numbers given in the IUPAC name. The prefix indicates the number of identical substituents in the molecule.
Answer to Problem A.44P
The structure for the given IUPAC name is as follows:
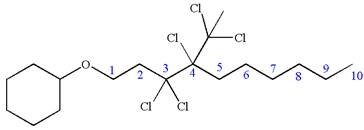
Explanation of Solution
The given IUPAC name is
The root name decane indicates the parent is a chain of ten carbon atoms.
![]()
Hence, the structure of
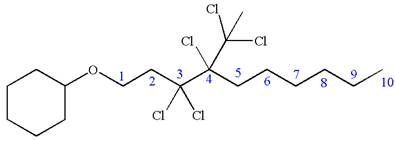
The structure for the given molecules is drawn from the root name, prefix and locator of substituent.
Want to see more full solutions like this?
Chapter A Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Assign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forward
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





