
a)

Interpretation:
How to convert 1-butyne in to butanone is to be shown.
Concept introduction:
Terminal
To show:
How to convert 1-butyne in to butanone.
b)
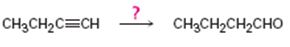
Interpretation:
How to convert 1-butyne in to butanal is to be shown.
Concept introduction:
Terminal alkynes when hydrated using hydroboration-oxidation reaction yield an enol with OH group attached to the terminal carbon as the reaction follows anti-Markovnikov regiochemistry. The enol obtained then tautomerizes to an
To show:
How to convert 1-butyne in to butanal is to be shown.
c)
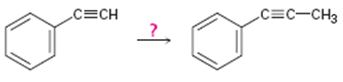
Interpretation:
How to convert ethynylbenzene in to propynylbenzene is to be shown.
Concept introduction:
Terminal alkynes are converted in to acetylides by treating with NaNH2 in liquid ammonia. The acetylides can be converted to higher alkynes by treating with
To show:
How to convert ethynylbenzene in to propynylbenzene.
d)
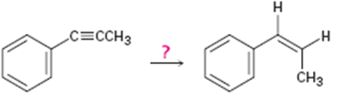
Interpretation:
How to convert propynylbenzene in to cis-1-propenylbenzene is to be shown.
Concept introduction:
Alkynes can be converted in to the corresponding
To show:
How to convert 1-propynylbenzene in to 1-propenylbenzene.
e)

Interpretation:
How to convert 1-butyne in to propanoic acid is to be shown.
Concept introduction:
Alkynes gets cleaved when treated with powerful oxidizing agents like KMnO4. Internal alkynes give carboxylic acids as products. Terminal alkynes give CO2 also along with a
To show:
How to convert 1-butyne in to propanoic acid.
f)
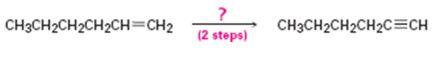
Interpretation:
How to convert 1-hexene in to 1-hexyne in two steps is to be shown.
Concept introduction:
Alkenes when treated with bromine yield a dibromo
To show:
How to convert 1-hexene in to 1-hexyne in two steps
Trending nowThis is a popular solution!

Chapter 9 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forward
- Can I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forward
- what is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


