
A Brayton cycle with regeneration using air as the working fluid has a pressure ratio of 7. The minimum and maximum temperatures in the cycle are 310 and 1150 K. Take an isentropic efficiency of 75 percent for the compressor and 82 percent for the turbine and an effectiveness of 65 percent for the regenerator. Determine the total exergy destruction associated with the cycle, assuming a source temperature of 1500 K and a sink temperature of 290 K. Also, determine the exergy of the exhaust gases at the exit of the regenerator. Use variable specific heats for air.
The exergy destruction associated with each process of the Brayton cycle and the exergy of the exhaust gases at the exit of the regenerator.
Answer to Problem 148P
The exergy destruction associated with process 1-2 of the given Brayton cycle is
The exergy destruction associated with process 3-4 of the given Brayton cycle is
The exergy destruction associated with regeneration process of the given Brayton cycle is
The exergy destruction associated with process 5-3 of the given Brayton cycle is
The exergy destruction associated with process 6-1 of the given Brayton cycle is
The exergy of the exhaust gases at the exit of the regenerator is
Explanation of Solution
Show the regenerative Brayton cycle with air as the working fluid, on
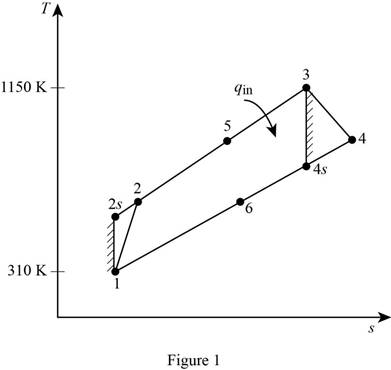
Consider, the pressure is
Write the pressure and relative pressure relation for the process 1-2.
Write the pressure and relative pressure relation for the process 3-4.
Write the expression of efficiency of the compressor
Write the expression of efficiency of the turbine
Write the expression of net work output by the gas turbine
Here, work done by the turbine is
Write the expression of effectiveness of the regenerator
Write the expression of heat input to the regenerative Brayton cycle
Write the expression of heat rejected by the regenerative Brayton cycle
Write the expression of thermal efficiency of the given turbine
Write the energy balance equation on the heat exchanger.
Write the expression of exergy destruction associated with the process 1-2 of the given Brayton cycle
Here, the temperature of the surroundings is
Write the expression of exergy destruction associated with the process 3-4 of the given Brayton cycle
Here, entropy of air at state 3 as a function of temperature is
Write the expression of exergy destruction associated with the regeneration process of the given Brayton cycle
Here, entropy of air at state 5 as a function of temperature alone is
Write the expression of exergy destruction associated with the process 5-3 of the given Brayton cycle
Here, the temperature of the heat source is
Write the expression of exergy destruction associated with the process 6-1 of the given Brayton cycle
Here, the temperature of the sink is
Write the expression of stream exergy at the exit of the regenerator (state 6)
Here, the specific enthalpy of the surroundings is
Write the expression of change entropy for the exit of the regenerator
Here, entropy of air at the surroundings as a function of temperature alone is
Conclusion:
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at a temperature of
Substitute 7 for
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at a relative pressure of 10.88
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at a temperature of
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the property of enthalpy
Rearrange Equation (III), and substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the property of entropy
Rearrange Equation (IV), and substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the property of entropy
Substitute
Substitute 0.65 for
Refer Table A-17, “Ideal gas properties of air”, obtain the property of entropy
Substitute
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at a enthalpy of
Substitute
Substitute
Substitute 290 K for
Thus, the exergy destruction associated with process 1-2 of the given Brayton cycle is
Substitute 290 K for
Thus, the exergy destruction associated with process 3-4 of the given Brayton cycle is
Substitute 290 K for
Thus, the exergy destruction associated with regeneration process of the given Brayton cycle is
Substitute 290 K for
Thus, the exergy destruction associated with process 5-3 of the given Brayton cycle is
Substitute 290 K for
Thus, the exergy destruction associated with process 6-1 of the given Brayton cycle is
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at a temperature of
At the exit of the regenerator, pressure remains constant,
Substitute
Substitute
Thus, the exergy of the exhaust gases at the exit of the regenerator is
Want to see more full solutions like this?
Chapter 9 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- This is an exam review question. The answer is Pmin = 622.9 lb but whyarrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardThis is an old practice exam. Fce = 110lb and FBCD = 62 lb but whyarrow_forwardQuiz/An eccentrically loaded bracket is welded to the support as shown in Figure below. The load is static. The weld size for weld w1 is h1 = 4mm, for w2 h2 = 6mm, and for w3 is h3 =6.5 mm. Determine the safety factor (S.f) for the welds. F=29 kN. Use an AWS Electrode type (E100xx). 163 mm S 133 mm 140 mm Please solve the question above I solved the question but I'm sure the answer is wrong the link : https://drive.google.com/file/d/1w5UD2EPDiaKSx3W33aj Rv0olChuXtrQx/view?usp=sharingarrow_forward
- Q2: (15 Marks) A water-LiBr vapor absorption system incorporates a heat exchanger as shown in the figure. The temperatures of the evaporator, the absorber, the condenser, and the generator are 10°C, 25°C, 40°C, and 100°C respectively. The strong liquid leaving the pump is heated to 50°C in the heat exchanger. The refrigerant flow rate through the condenser is 0.12 kg/s. Calculate (i) the heat rejected in the absorber, and (ii) the COP of the cycle. Yo 8 XE-V lo 9 Pc 7 condenser 5 Qgen PG 100 Qabs Pe evaporator PRV 6 PA 10 3 generator heat exchanger 2 pump 185 absorberarrow_forwardQ5:(? Design the duct system of the figure below by using the balanced pressure method. The velocity in the duct attached to the AHU must not exceed 5m/s. The pressure loss for each diffuser is equal to 10Pa. 100CFM 100CFM 100CFM ☑ ☑ 40m AHU -16m- 8m- -12m- 57m 250CFM 40m -14m- 26m 36m ☑ 250CFMarrow_forwardA mass of ideal gas in a closed piston-cylinder system expands from 427 °C and 16 bar following the process law, pv1.36 = Constant (p times v to the power of 1.36 equals to a constant). For the gas, initial : final pressure ratio is 4:1 and the initial gas volume is 0.14 m³. The specific heat of the gas at constant pressure, Cp = 0.987 kJ/kg-K and the specific gas constant, R = 0.267 kJ/kg.K. Determine the change in total internal energy in the gas during the expansion. Enter your numerical answer in the answer box below in KILO JOULES (not in Joules) but do not enter the units. (There is no expected number of decimal points or significant figures).arrow_forward
- my ID# 016948724. Please solve this problem step by steparrow_forwardMy ID# 016948724 please find the forces for Fx=0: fy=0: fz=0: please help me to solve this problem step by steparrow_forwardMy ID# 016948724 please solve the proble step by step find the forces fx=o: fy=0; fz=0; and find shear moment and the bending moment diagran please draw the diagram for the shear and bending momentarrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

