
Concept explainers
The total exergy destruction for each process of an ideal dual cycle
Answer to Problem 145P
The total exergy destruction in an ideal dual cycle is
Explanation of Solution
Draw the ideal dual cycle on
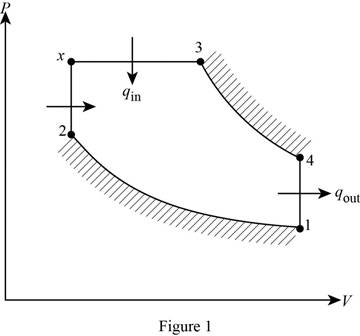
Consider, the pressure is
Write the expression of temperature and volume relation for the isentropic compression process 1-2.
Here, compression ratio is r and specific heat ratio is k.
Write the expression of pressure, and volume relation for the isentropic expansion process 1-2.
Write the expression of pressure ratio relation.
Here, pressure ratio is
Write the expression of temperature, and pressure relation for the constant volume heat addition process
Write the expression of temperature, and volume relation for the constant pressure heat addition process
Here, cutoff ratio is
Write the expression of temperature, and volume relation for the constant pressure heat addition process 3-4.
Write the expression to calculate the heat added to the cycle during process
Write the expression to calculate the heat added to the cycle during process
Here, heat input to the process
Write the expression to calculate the heat added to the cycle during process
Here, specific heat at constant pressure is
Write the expression of net heat addition to the cycle
Write the expression for exergy destruction during the process of the cycle.
Here, temperature of the surroundings is
Write the expression of entropy change for the process
Here, specific heat of air at constant volume is
Write the expression for the exergy loss for the isothermal process
Write the expression of entropy change for the isothermal process
Write the expression for the exergy loss for the process
Write the expression of entropy change for the process
Write the expression for the exergy loss for the process
Here, temperature of the sink is
Write the expression to calculate the total in an ideal dual cycle.
Conclusion:
From Table A-1E, “Molar mass, gas constant, and critical-point properties”, obtain the following properties of air at room temperature.
From Table A-2Ea, “Ideal-gas specific heats of various common gases”, obtain the value for gas content
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Equation (VII).
The exergy loss for the isothermal process 1-2
Here
Substitute
Substitute
Substitute
Substitute
The exergy loss for the isothermal process 3-4
Here,
Substitute
Substitute
During heat rejection process the largest exergy destruction in an ideal dual cycle occur.
Substitute
Thus, the total exergy destruction in an ideal dual cycle is
Want to see more full solutions like this?
Chapter 9 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- Just do Questions 7, 9, 11. Here are notes attached for reference. I prefer handwritten solutions. ONLY UPLOAD A SOLUTION IF YOU ARE SURE ABOUT THE ANSWER PLEASE.arrow_forwardExample Consider the simplified model of an automobile shown in Figure, let the parameter have the values m = 1500 kg, Ic = 2000 kg m2, k₁ = 36000 kg/m, k₁ = 40000 kg/m, a = 1.3 m and b = 1.7 m, calculate the natural modes of the system and write the expression for the response. ט 0 Solution T Equilibrium position 6(1)arrow_forward6.3 Contamination of vegetable oil Vegetable oil is used in a food processing factory to prepare instant breadcrumbs. A stirred tank is used to hold the oil. During operation of the breadcrumb process, oil is pumped from the tank at a rate of 4.8 1 h-¹. At 8 PM during the night shift, the tank is mistakenly connected to a drum of cod liver oil, which is then pumped into the tank. The volume of vegetable oil in the tank at 8 PM is 60 1. (a) If the flow rate of cod liver oil into the tank is 7.5 1h-1 and the tank has a maximum capacity of 100 1, will the tank overflow before the factory manager arrives at 9 AM? Assume that the density of both oils is the same. (b) If cod liver oil is pumped into the tank at a rate of 4.8 1 h 1 instead of 7.5 1 h−1, what is the composition of oil in the tank at midnight?arrow_forward
- 6.1 Dilution of sewage In a sewage treatment plant, a large concrete tank initially contains 440,000 litres of liquid and 10,000 kg of fine suspended solids. To flush this material out of the tank, water is pumped into the vessel at a rate of 40,000 1 h −1. Liquid containing solids leaves at the same rate. Estimate the concentration of suspended solids in the tank at the end of 5 h. State your assumptions.arrow_forward6.13 Heating glycerol solution An adiabatic stirred tank is used to heat 100 kg of a 45% glycerol solution in water. An electrical coil delivers 2.5 kW of power to the tank; 88% of the energy delivered by the coil goes into heating the vessel contents. The glycerol solution is initially at 15°C. (a) Write a differential equation for the energy balance. (b) Integrate the equation to obtain an expression for temperature as a function of time. (c) Assuming glycerol and water form an ideal solution, how long will the solution take to reach 90°C?arrow_forwardI don't see any other way to try to solve this problem can you please help me out with this?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





