
Concept explainers
(a)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(a)
Answer to Problem 9.90P
Explanation of Solution
The given chemical equation for the formation of dimethyl ether
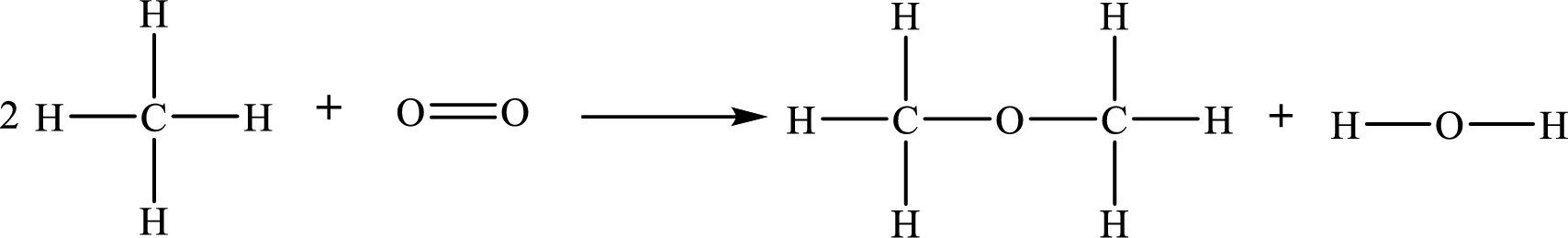
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
The given chemical equation for the formation of ethanol
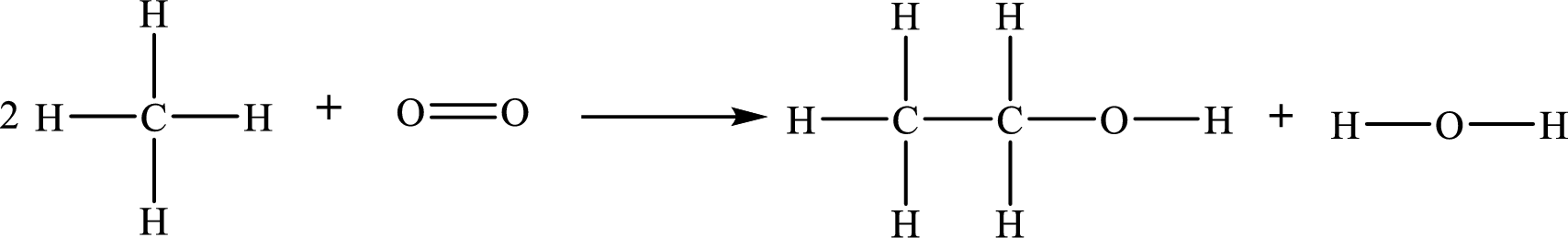
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
(b)
Interpretation:
Among the
Concept introduction:
In the case of a reaction, the change in enthalpy
Here,
Endothermic reactions are the reactions in which energy in the form of the heat or light is absorbed by the reactant for the formation of the product.
Exothermic reactions are the reactions in which energy in the form of the heat or light is released with the product.
(b)
Answer to Problem 9.90P
The formation reaction of ethanol is more exothermic as compared to dimethyl ether.
Explanation of Solution
The value of
Exothermic reactions are the reactions in which energy in the form of the heat or light is released with the product.
(c)
Interpretation:
Concept introduction:
Hess’s law is used to calculate the enthalpy change of an overall reaction that can be derived as a sum of two or more reaction. According to Hess’s law
Enthalpy is a state function so the value depends upon the initial state and final state not on the path so
(c)
Answer to Problem 9.90P
Explanation of Solution
The enthalpy change of the following reaction is
The enthalpy change of the following reaction is
Reverse the equation (2).
The enthalpy change for the reaction (3) is calculated as,
Add equation (1) and (3).
The enthalpy change of the final reaction (4) is
The expression to calculate
Substitute
Want to see more full solutions like this?
Chapter 9 Solutions
Connect 2-Year Access Card for Chemistry: The Molecular Nature of Matter and Change
- Please help me answer a. Please and thank you I advance.arrow_forwardDraw both of the chair flips for both the cis and trans isomers for the following compounds: 1,4-diethylcyclohexane 1-methyl-3-secbutylcyclohexanearrow_forwardPpplllleeeaaasssseeee hellppp wiithhh thisss physical chemistryyyyy I talked like this because AI is very annoyingarrow_forward
- For this question, if the product is racemic, input both enantiomers in the same Marvin editor. A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a racemic product. Input 1 for Cl, in the cold and dark Input 2 for Oy followed by H₂O, Zn Input 3 for D₂ with metal catalyst Input 4 for H₂ with metal catalyst B) Draw the skeletal structure of the major organic product made from the reagent in part A Marvin JS Help Edit drawing C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with peroxyacetic acid. Marvin 35 Helparrow_forwardMichael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





