
Concept explainers
(a)
Interpretation:
The condensed electron configurations and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(a)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
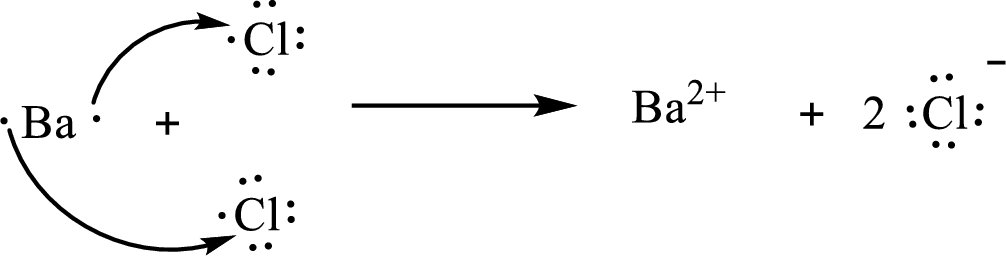
The Lewis orbital diagram is as follows:
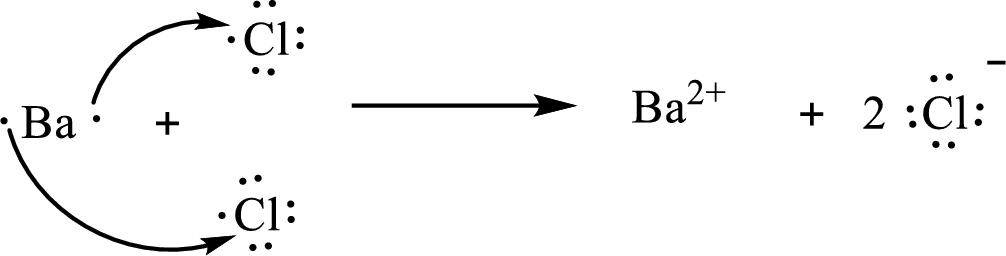
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a barium atom
The condensed electronic configuration of the chlorine atom
Barium atom loses two electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Barium atom loses two electrons to form
The Lewis orbital diagram is as follows:
(b)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(b)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
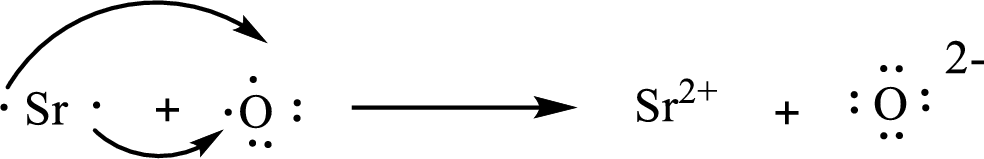
The Lewis orbital diagram is as follows:
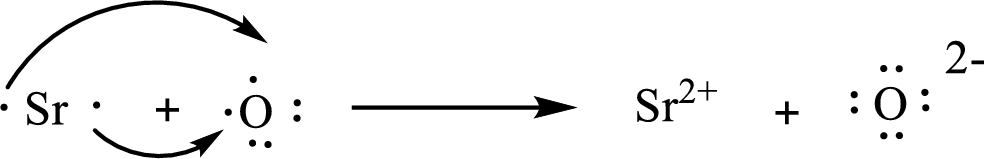
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a strontium atom
The condensed electronic configuration of oxygen atom
Strontium atom loses two electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Strontium atom loses two electrons to form
The Lewis orbital diagram is as follows:
(c)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(c)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
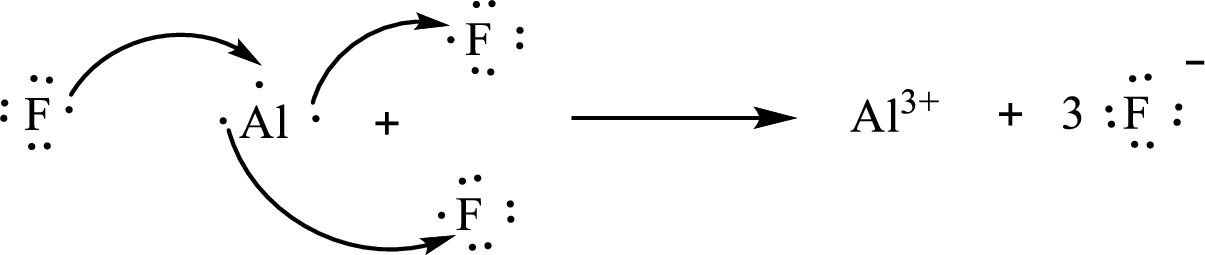
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of an aluminium atom
The condensed electronic configuration of the fluorine atom
Aluminium atom loses three electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Aluminium atom loses three electrons to form
The Lewis orbital diagram is as follows:
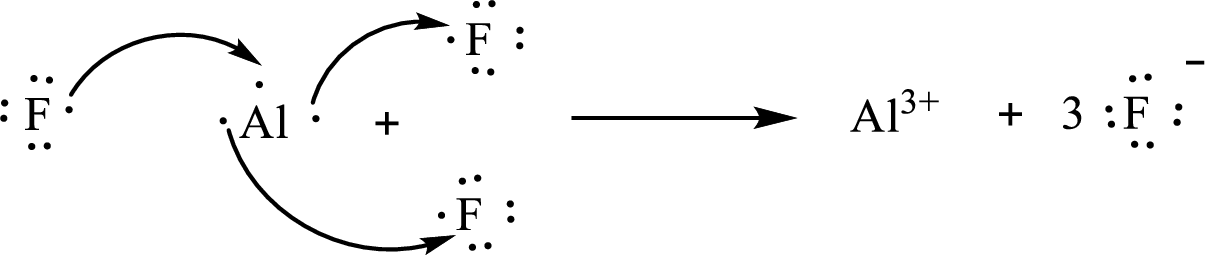
(d)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(d)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
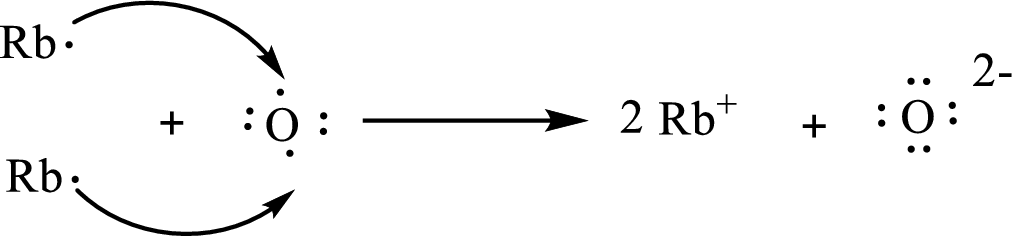
The Lewis orbital diagram is as follows:
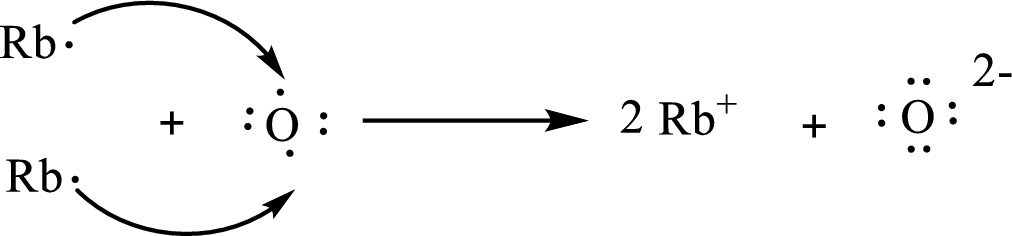
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a rubidium atom
The condensed electronic configuration of oxygen atom
Two rubidium atoms lose one electron respectively to form
The condensed electronic configuration of
The condensed electronic configuration of
Two rubidium atoms lose one electron respectively to form
The Lewis orbital diagram is as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
Connect 2-Year Access Card for Chemistry: The Molecular Nature of Matter and Change
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
- Ppplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





