
Concept explainers
Interpretation:
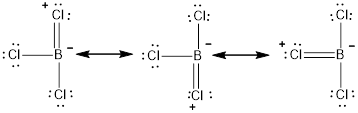
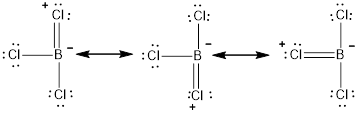
The three resonance structures for the given molecule
Concept Introduction:
Octet rule: According to octet rule, when an atom contains eight electrons in its valence shell (outermost shell), it will be stable. Most of the atom will donate or accept electrons to its outer most orbital to satisfy the octet rule.
The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Sometimes the
All the possible resonance structures are imaginary whereas the resonance hybrid is real.
Resonance is a method used to describe about delocalized electrons inside certain molecules or polyatomic ions since the Lewis structure can’t express it. A molecule or ion containing delocalized electrons can be represented by using several similar structures such structures are called as resonance structures or canonical structures.
The general rules to be followed for drawing resonance structures are given below:
- 1. The position of the atoms does not change only the pi electrons and a lone pair of electrons can change their positions.
- 2. The total number of valence electrons in all the resonance structures remains the same.
- 3. Octet rule must not be violated. No atom can have electrons more than 8 in its valence shell except sulphur.
- 4. Transfer of electrons between the bonds is shown by curved arrows.
Answer to Problem 9.59QP
The resonance structure with the formal charges is represented as follows,
Explanation of Solution
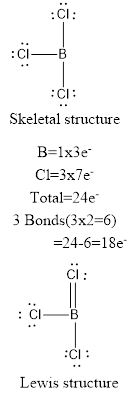
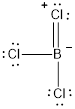
The molecule of
The formal charges for atoms in the resonance structures are calculated as follows,
- Boron atom
Substituting these values to the equation,
- Chlorine atom in double bond
Substituting these values to the equation,
- Chlorine atom in both single bond
Substituting these values to the equation,
- Boron atom
Substituting these values to the equation,
- Chlorine atom in double bond
Substituting these values to the equation,
- Chlorine atom in both single bonds
Substituting these values to the equation,
- Boron atom
Substituting these values to the equation,
- Chlorine atom in double bond
Substituting these values to the equation,
- Chlorine atom in both single bond
Substituting these values to the equation,
The three resonance structures for the given molecule
Want to see more full solutions like this?
Chapter 9 Solutions
Connect 1 Semester Access Card for General Chemistry: The Essential Concepts
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





