
CHEMISTRY(LOOSELEAF) W/CONNECT+EBOOK
7th Edition
ISBN: 9781259712500
Author: SILBERBERG
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Question
Chapter 9, Problem 9.55P
Interpretation Introduction
Interpretation:
The relation between the partial ionic character of a bond in a diatomic molecule and
Concept introduction:
Electronegativity is the tendency of an atom to attract the shared electrons in the bond towards itself. The more electronegative atom will more attract the bonding electrons towards itself than the less electronegative atom. Therefore the electrons will spend more time with the more electronegative atom than an electropositive atom. The electronegative atom will acquire the partial negative charge and the electropositive atom will acquire a partial positive charge.
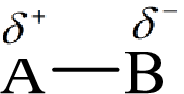
Here, B is the electronegative atom and A is the electropositive atom.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.
How many neighbors does the proton that produces the multiplet below have?
2.
3.
اللـ
Draw a partial structure from the multiplet below. (The integration of the multiplet is 6)
M
Using the additivity constants found in appendix G of your lab manual, calculate the approximate chemical
shifts of the protons indicated below. (Show your work!!!)
B
A
Br
SH
1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°?
To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide.
kindly show me how to solve this long problem. Thanks
4.
An 'H-NMR of a compound is acquired. The integration for signal A is 5692 and the integration for signal
B is 25614. What is the simplest whole number ratio of protons for signals A and B? (Show your work!!!)
5.
Assign the carbons in the NMR below as either carbonyl, aromatic, or alkyl.
200
150
100
50
ō (ppm)
1
Chapter 9 Solutions
CHEMISTRY(LOOSELEAF) W/CONNECT+EBOOK
Ch. 9.2 - Use condensed electron configurations, partial...Ch. 9.2 - Prob. 9.1BFPCh. 9.2 - Prob. 9.2AFPCh. 9.2 - Prob. 9.2BFPCh. 9.3 - Prob. 9.3AFPCh. 9.3 - Prob. 9.3BFPCh. 9.3 - Prob. B9.1PCh. 9.3 - Prob. B9.2PCh. 9.4 - One of the most important industrial reactions is...Ch. 9.4 - Prob. 9.4BFP
Ch. 9.5 - Arrange each set of bonds in order of increasing...Ch. 9.5 - Prob. 9.5BFPCh. 9 - Prob. 9.1PCh. 9 - Prob. 9.2PCh. 9 - What is the relationship between the tendency of a...Ch. 9 - Prob. 9.4PCh. 9 - Prob. 9.5PCh. 9 - State the type of bonding—ionic, covalent, or...Ch. 9 - State the type of bonding—ionic, covalent, or...Ch. 9 - State the type of bonding—ionic, covalent, or...Ch. 9 - Prob. 9.9PCh. 9 - Prob. 9.10PCh. 9 - Prob. 9.11PCh. 9 - Prob. 9.12PCh. 9 - Prob. 9.13PCh. 9 - Give the group number and condensed electron...Ch. 9 - Give the group number and condensed electron...Ch. 9 - Prob. 9.16PCh. 9 - Prob. 9.17PCh. 9 - Prob. 9.18PCh. 9 - Prob. 9.19PCh. 9 - Prob. 9.20PCh. 9 - Prob. 9.21PCh. 9 - Prob. 9.22PCh. 9 - Prob. 9.23PCh. 9 - Prob. 9.24PCh. 9 - Prob. 9.25PCh. 9 - For each pair, choose the compound with the larger...Ch. 9 - Prob. 9.27PCh. 9 - For each pair, choose the compound with the...Ch. 9 - Prob. 9.29PCh. 9 - Use the following to calculate of NaCl:
Compared...Ch. 9 - Use the following to calculate of MgF2:
Compared...Ch. 9 - Prob. 9.32PCh. 9 - Born-Haber cycles were used to obtain the first...Ch. 9 - Prob. 9.34PCh. 9 - Prob. 9.35PCh. 9 - Prob. 9.36PCh. 9 - How does the energy of the bond between a given...Ch. 9 - When liquid benzene (C6H6) boils, does the gas...Ch. 9 - Prob. 9.39PCh. 9 - Prob. 9.40PCh. 9 - Prob. 9.41PCh. 9 - Prob. 9.42PCh. 9 - The text points out that, for similar types of...Ch. 9 - Why is there a discrepancy between an enthalpy of...Ch. 9 - Which of the following gases would you expect to...Ch. 9 - Which of the following gases would you expect to...Ch. 9 - Use bond energies to calculate the enthalpy of...Ch. 9 - Prob. 9.48PCh. 9 - Prob. 9.49PCh. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - What is the general relationship between IE1 and...Ch. 9 - Is the H—O bond in water nonpolar covalent, polar...Ch. 9 - Prob. 9.54PCh. 9 - How is the partial ionic character of a bond in a...Ch. 9 - Using the periodic table only, arrange the...Ch. 9 - Prob. 9.57PCh. 9 - Prob. 9.58PCh. 9 - Prob. 9.59PCh. 9 - Prob. 9.60PCh. 9 - Use Figure 9.21 to indicate the polarity of each...Ch. 9 - Prob. 9.62PCh. 9 - Prob. 9.63PCh. 9 - Prob. 9.64PCh. 9 - Prob. 9.65PCh. 9 - Prob. 9.66PCh. 9 - Prob. 9.67PCh. 9 - Prob. 9.68PCh. 9 - Prob. 9.69PCh. 9 - Prob. 9.70PCh. 9 - Prob. 9.71PCh. 9 - Geologists have a rule of thumb: when molten rock...Ch. 9 - Prob. 9.73PCh. 9 - Use Lewis electron-dot symbols to represent the...Ch. 9 - Prob. 9.75PCh. 9 - Prob. 9.76PCh. 9 - By using photons of specific wavelengths, chemists...Ch. 9 - Prob. 9.78PCh. 9 - Prob. 9.79PCh. 9 - Prob. 9.80PCh. 9 - Prob. 9.81PCh. 9 - Prob. 9.82PCh. 9 - Prob. 9.83PCh. 9 - Find the longest wavelengths of light that can...Ch. 9 - The work function (ϕ) of a metal is the minimum...Ch. 9 - Prob. 9.86PCh. 9 - Prob. 9.87PCh. 9 - Prob. 9.88PCh. 9 - In a future hydrogen-fuel economy, the cheapest...Ch. 9 - Prob. 9.90PCh. 9 - Prob. 9.91P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Speaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forwardIf we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forwardWhen natural light falls perpendicularly on a material A, it has a reflectivity of 0.813%. Indicate the value of the refractive index.arrow_forward
- In piezoelectricity and piezoelectric ceramics, one of the following options is false:(A). Piezoelectricity allows an electrical signal to be transformed into a mechanical one.(B). PbZrO3 is a well-known piezoelectric ceramic.(C). Piezoelectricity and ferroelectricity in general have no relationship.(D). One of the applications of piezoelectricity is sonar.arrow_forward(30 MARKS) Give the major product(s ) formed including relevant stereochemistry or the complete reaction conditions for the following reactions. More than one step may be required for each reaction arrow, in which case the steps must be numbered 1), 2) etc. (2 marks each box) h) i) h) OH i) HO H3PO4, heat 2 Brarrow_forwardNonearrow_forward
- Indicate which option is false(A). Resistivity has a residual component and a thermal component.(B). In some materials resistivity increases with T and in others it decreases.(C). In insulating materials, resistivity is very low.arrow_forwardIn ceramic materials, in relation to polymorphism, the same substance crystallizes differently when external conditions vary. Is this correct?arrow_forwardIndicate the type of bond that is considered to be a hydrogen bond.(A). Permanent dipole-dipole interaction between polar molecules.(B). Mixed ionic-covalent bond.(C). Principal interatomic bond(D). Van del Waals forces.arrow_forward
- Retro aldol: NaOH H₂O H NaOH & d H₂O Harrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. H conc. HBr Drawing Qarrow_forwardCalculate the atomic packing factor of diamond knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are, respectively, 0.038 and 0.117 nm.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY