
You are checking the performance of a reactor in which acetylene is produced from methane in the reaction
An undesired side reaction is the decomposition of acetylene:
Methane is fed to the reactor at 1500°C at a rate of 10.0 mol CH4/S. Heat is transferred to the reactor at a rate of 975 kW. The product temperature is 1500°C and the fractional conversion of methane is 0.600. A flowchart of the process and an enthalpy table are shown below.
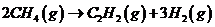
References: C(s), H2(g), at 25°C, 1 atm
| Substance |
|
|
|
|
|
|
10.0 | 41.65 |
|
|
|
|
— | — |
|
|
|
|
— | — |
|
|
| C | — | — |
|
|
(a) Using the heat capacities given below for enthalpy calculations, write and solve material balances and an energy balance to determine the product component flow rates and the yield of acetylene (mol C2H2produced/mol CH4consumed).
For example, the specific enthalpy of methane at 1500°C relative to methane at 25°C is [0.079 kJ/(mol·°C)](1500°C - 25°C) = 116.5 kJ/mol.
(b) The reactor efficiency may be defined as the ratio (actual acetylene yield/acetylene yield with no side reaction). What is the reactor efficiency for this process?
(c) The mean residence time in the reactor [
If the mean residence time increases to infinity, what would you expect to find in the product stream? Explain.
Someone proposes running the process with a much greater feed rate than the one used in Part (a), separating the products from the unconsumed reactants, and recycling the reactants. Why would you expect that process design to increase the reactor efficiency? What else would you need to know to determine whether the new design would be cost-effective?
Trending nowThis is a popular solution!

Chapter 9 Solutions
ELEM.PRINCIPLES OF CHEMICAL PROCESSES
Additional Engineering Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Digital Fundamentals (11th Edition)
Electric Circuits (10th Edition)
Mechanics of Materials
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





