
Assume that two liquids are separated by a semipermeable membrane, with pure solvent on the right side and a solution of a solute on the left side. Make a drawing that shows the situation after equilibrium is reached.
Interpretation:
The situation after equilibrium is reached when two liquids are separated by a semipermeable membrane has to be drawn.
Concept introduction:
Colligative properties such as vapor pressure, freezing point, and boiling point are affected by the presence of solute particles in a solution.
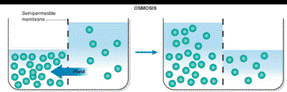
Osmosis is the passage of substances in true solution through a semipermeable membrane.
Particles such as starches and protein molecules are too large to pass the membrane.
The restriction of passage of large particles causes the smaller particles to pass more rapidly in the direction of higher concentrations, producing an osmotic pressure in confined liquids.
Explanation of Solution
Osmosis occurs when solution of different concentration are separated by a semipermeable membrane that allows solvent molecules to pass but blocks the passage of solute ions and molecules. Solvent flows from the more dilute side to the more concentrated side until sufficient osmotic pressure builds up and stops the flows. An effect similar to osmosis occurs when membranes of large pore size are used.
The situation after equilibrium is reached when two liquids are separated by a semipermeable membrane is drawn as
Want to see more full solutions like this?
Chapter 9 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
- Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5arrow_forwardShow a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forward
- Don't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forwardhow would you make this plot in excel?arrow_forwardwhat is the productarrow_forward
- Balance the following equation and list of coefficients in order from left to right. SF4+H2O+—-> H2SO3+HFarrow_forwardProblem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





