
Concept explainers
(a)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(a)
Answer to Problem 9.13P
The structural formula for the product of given

Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

(b)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(b)
Answer to Problem 9.13P
The structural formula for the product of given
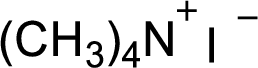
Explanation of Solution
In the given
In this reaction formed quaternary ammonium ion is stabilized by iodine ion so the product is an iodine salt of quaternary ammonium ion.
Given reactant is non-chiral so product also non-chiral.

(c)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(c)
Answer to Problem 9.13P
The structural formula for the product of given
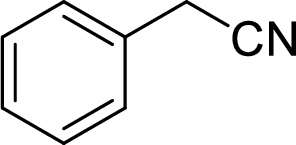
Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.
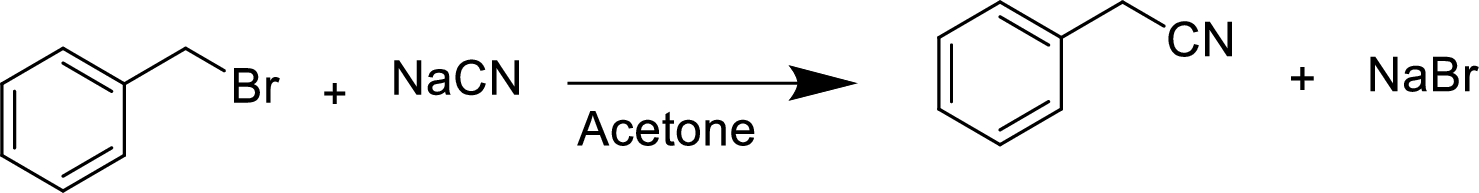
(d)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(d)
Answer to Problem 9.13P
The structural formula for the product of given
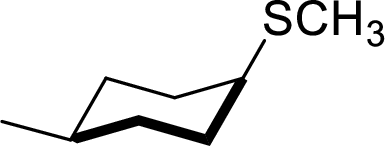
Explanation of Solution
In the given
Given reactant has an equatorial chlorine hence, the product is axial.
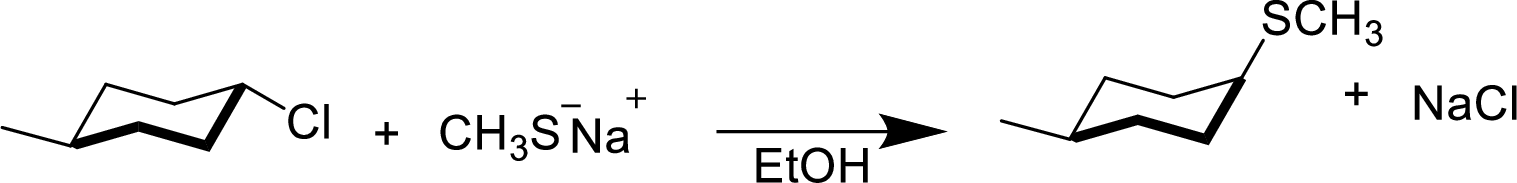
(e)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(e)
Answer to Problem 9.13P
The structural formula for the product of given
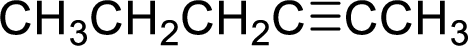
Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

(f)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(f)
Answer to Problem 9.13P
The structural formula for the product of given
Explanation of Solution
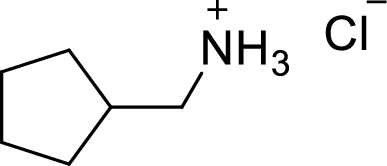
In the given
In this reaction formed ammonium ion is stabilized by chorine ion so the product is a chlorine salt of ammonium ion.
Given reactant is non-chiral so product also non-chiral.
(g)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(g)
Answer to Problem 9.13P
The structural formula for the product of given
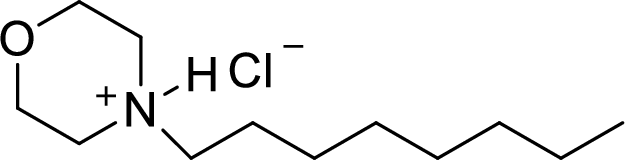
Explanation of Solution
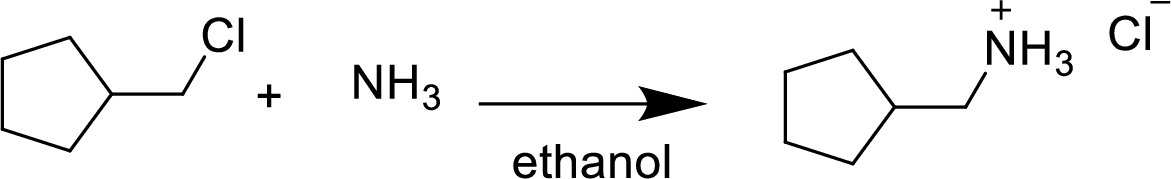
In the given
In this reaction formed ammonium ion is stabilized by chorine ion so the product is a chlorine salt of ammonium ion.
Given reactant is non-chiral so product also non-chiral.

(h)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(h)
Answer to Problem 9.13P
The structural formula for the product of given

Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- what is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forwardDraw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



