
CHEMISTRY:ATOMS FIRST-2 YEAR CONNECT
2nd Edition
ISBN: 9781260592320
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.11QP
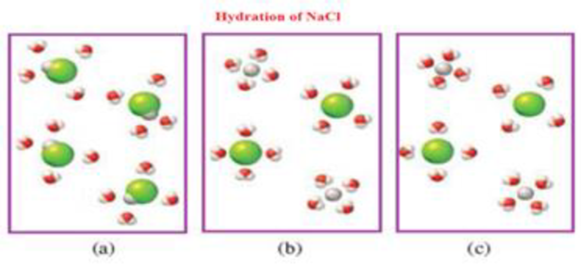
Which of the following diagrams best represents the hydration of NaCl when dissolved in water? The Cl− ion is larger in size than the Na+ ion.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please
please help me please please
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
N2 (g) + 3H2 (g) = 2NH3 (g) AG⁰ = -34. KJ
Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this
system:
?
rise
Under these conditions, will the pressure of N2 tend to rise or fall?
☐ x10
fall
Is it possible to reverse this tendency by adding H₂?
In other words, if you said the pressure of N2 will tend to rise, can that be
changed to a tendency to fall by adding H₂? Similarly, if you said the
pressure of N2 will tend to fall, can that be changed to a tendency to rise
by adding H₂?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of H₂ needed to reverse it.
Round your answer to 2 significant digits.
yes
no
☐
atm
☑
5
00.
18
Ar
Chapter 9 Solutions
CHEMISTRY:ATOMS FIRST-2 YEAR CONNECT
Ch. 9.1 - Sports drinks typically contain sucrose...Ch. 9.1 - Prob. 1PPACh. 9.1 - Prob. 1PPBCh. 9.1 - Prob. 1PPCCh. 9.1 - Prob. 9.1.1SRCh. 9.1 - Prob. 9.1.2SRCh. 9.1 - Prob. 9.1.3SRCh. 9.2 - Classify each of the following compounds as...Ch. 9.2 - Prob. 2PPACh. 9.2 - Prob. 2PPB
Ch. 9.2 - Using Tables 9.2 and 9.3, identify a compound that...Ch. 9.2 - Prob. 9.3WECh. 9.2 - Prob. 3PPACh. 9.2 - Prob. 3PPBCh. 9.2 - Which diagram best represents the result when...Ch. 9.2 - Prob. 9.2.1SRCh. 9.2 - Prob. 9.2.2SRCh. 9.2 - Prob. 9.2.3SRCh. 9.2 - Prob. 9.2.4SRCh. 9.2 - Prob. 9.2.5SRCh. 9.3 - Prob. 9.4WECh. 9.3 - Prob. 4PPACh. 9.3 - Prob. 4PPBCh. 9.3 - Prob. 4PPCCh. 9.3 - Prob. 9.3.1SRCh. 9.3 - Prob. 9.3.2SRCh. 9.3 - Prob. 9.3.3SRCh. 9.3 - Prob. 9.3.4SRCh. 9.4 - Prob. 9.5WECh. 9.4 - Prob. 5PPACh. 9.4 - Prob. 5PPBCh. 9.4 - Write the balanced equation for the reaction...Ch. 9.4 - Prob. 9.6WECh. 9.4 - Using the activity series, predict which of the...Ch. 9.4 - Prob. 6PPBCh. 9.4 - Prob. 6PPCCh. 9.4 - Prob. 9.7WECh. 9.4 - Predict which of the following reactions will...Ch. 9.4 - Prob. 7PPBCh. 9.4 - Prob. 7PPCCh. 9.4 - Prob. 9.4.1SRCh. 9.4 - Prob. 9.4.2SRCh. 9.4 - Prob. 9.4.3SRCh. 9.4 - Prob. 9.4.4SRCh. 9.5 - Prob. 9.8WECh. 9.5 - Prob. 8PPACh. 9.5 - Prob. 8PPBCh. 9.5 - Prob. 8PPCCh. 9.5 - Prob. 9.9WECh. 9.5 - Prob. 9PPACh. 9.5 - Prob. 9PPBCh. 9.5 - Prob. 9PPCCh. 9.5 - Starting with a 2.0-M stock solution of...Ch. 9.5 - Starting with a 6.552-M stock solution of HNO3,...Ch. 9.5 - Five standard solutions of HBr are prepared by...Ch. 9.5 - Prob. 10PPCCh. 9.5 - Prob. 9.11WECh. 9.5 - Prob. 11PPACh. 9.5 - Prob. 11PPBCh. 9.5 - Prob. 11PPCCh. 9.5 - Prob. 9.12WECh. 9.5 - Calculate the hydronium ion concentration in a...Ch. 9.5 - Prob. 12PPBCh. 9.5 - Prob. 12PPCCh. 9.5 - Prob. 9.13WECh. 9.5 - Prob. 13PPACh. 9.5 - Prob. 13PPBCh. 9.5 - Prob. 13PPCCh. 9.5 - Prob. 9.5.1SRCh. 9.5 - Prob. 9.5.2SRCh. 9.5 - Prob. 9.5.3SRCh. 9.5 - Prob. 9.5.4SRCh. 9.5 - Prob. 9.5.5SRCh. 9.5 - Prob. 9.5.6SRCh. 9.6 - Prob. 9.14WECh. 9.6 - Prob. 14PPACh. 9.6 - Prob. 14PPBCh. 9.6 - Which diagram best represents the solution...Ch. 9.6 - Prob. 9.15WECh. 9.6 - Prob. 15PPACh. 9.6 - What volume (in mL) of a 0.2550 M NaOH solution...Ch. 9.6 - Prob. 15PPCCh. 9.6 - Prob. 9.16WECh. 9.6 - Prob. 16PPACh. 9.6 - Prob. 16PPBCh. 9.6 - Prob. 16PPCCh. 9.6 - Prob. 9.17WECh. 9.6 - Prob. 17PPACh. 9.6 - What is the molar mass of a diprotic acid if 30.5...Ch. 9.6 - Prob. 17PPCCh. 9.6 - Prob. 9.6.1SRCh. 9.6 - Prob. 9.6.2SRCh. 9.6 - Prob. 9.6.3SRCh. 9.6 - Prob. 9.6.4SRCh. 9 - Define solute, solvent, and solution by describing...Ch. 9 - What is the difference between a nonelectrolyte...Ch. 9 - Prob. 9.3QPCh. 9 - Prob. 9.4QPCh. 9 - Prob. 9.5QPCh. 9 - Prob. 9.6QPCh. 9 - You are given a water-soluble compound X. Describe...Ch. 9 - Prob. 9.8QPCh. 9 - Prob. 9.9QPCh. 9 - Prob. 9.10QPCh. 9 - Which of the following diagrams best represents...Ch. 9 - Prob. 9.12QPCh. 9 - Prob. 9.13QPCh. 9 - Describe hydration. What properties of water...Ch. 9 - What is the difference between a molecular...Ch. 9 - Prob. 9.16QPCh. 9 - Prob. 9.17QPCh. 9 - Prob. 9.18QPCh. 9 - Which reaction is represented by the net ionic...Ch. 9 - Prob. 9.20QPCh. 9 - Characterize the following compounds as soluble or...Ch. 9 - Write ionic and net ionic equations for the...Ch. 9 - Write ionic and net ionic equations for the...Ch. 9 - Prob. 9.24QPCh. 9 - Which of the following processes will likely...Ch. 9 - List the general properties of acids and bases.Ch. 9 - Prob. 9.27QPCh. 9 - Prob. 9.28QPCh. 9 - Prob. 9.29QPCh. 9 - What factors qualify a compound as a salt? Specify...Ch. 9 - Identify the following as a weak or strong acid or...Ch. 9 - Prob. 9.32QPCh. 9 - Prob. 9.33QPCh. 9 - Prob. 9.34QPCh. 9 - Prob. 9.35QPCh. 9 - Prob. 9.36QPCh. 9 - Prob. 9.37QPCh. 9 - Prob. 9.38QPCh. 9 - Describe how the activity series is organized, and...Ch. 9 - Prob. 9.40QPCh. 9 - Prob. 9.41QPCh. 9 - For the complete redox reactions represented here,...Ch. 9 - Prob. 9.43QPCh. 9 - Prob. 9.44QPCh. 9 - Prob. 9.45QPCh. 9 - Prob. 9.46QPCh. 9 - Give the oxidation numbers for the underlined...Ch. 9 - Give the oxidation numbers for the underlined...Ch. 9 - Prob. 9.49QPCh. 9 - Prob. 9.50QPCh. 9 - Prob. 9.51QPCh. 9 - Prob. 9.52QPCh. 9 - Prob. 9.53QPCh. 9 - Prob. 9.54QPCh. 9 - Prob. 9.55QPCh. 9 - Which of the following would result in the actual...Ch. 9 - Why cant we prepare the solution by first filling...Ch. 9 - Prob. 9.3VCCh. 9 - Prob. 9.4VCCh. 9 - Prob. 9.56QPCh. 9 - Prob. 9.57QPCh. 9 - Prob. 9.58QPCh. 9 - Prob. 9.59QPCh. 9 - Prob. 9.60QPCh. 9 - Prob. 9.61QPCh. 9 - Prob. 9.62QPCh. 9 - Prob. 9.63QPCh. 9 - Prob. 9.64QPCh. 9 - Prob. 9.65QPCh. 9 - Prob. 9.66QPCh. 9 - Prob. 9.67QPCh. 9 - Prob. 9.68QPCh. 9 - Prob. 9.69QPCh. 9 - Prob. 9.70QPCh. 9 - Prob. 9.71QPCh. 9 - Prob. 9.72QPCh. 9 - Prob. 9.73QPCh. 9 - Prob. 9.74QPCh. 9 - Prob. 9.75QPCh. 9 - Prob. 9.76QPCh. 9 - Prob. 9.77QPCh. 9 - Prob. 9.78QPCh. 9 - Prob. 9.79QPCh. 9 - Prob. 9.80QPCh. 9 - Prob. 9.81QPCh. 9 - Prob. 9.82QPCh. 9 - Complete the following table for a solution at...Ch. 9 - (a) What is the Na+ concentration in each of the...Ch. 9 - (a) Determine the chloride ion concentration in...Ch. 9 - Prob. 9.86QPCh. 9 - Determine the resulting nitrate ion concentration...Ch. 9 - Prob. 9.88QPCh. 9 - Absorbance values for five standard solutions of a...Ch. 9 - Which best represents the before-and-after...Ch. 9 - Prob. 9.91QPCh. 9 - Describe the basic steps involved in gravimetric...Ch. 9 - Explain why distilled water must be used in the...Ch. 9 - Describe the basic steps involved in an acid-base...Ch. 9 - Prob. 9.95QPCh. 9 - Prob. 9.96QPCh. 9 - Would the volume of a 0.10 M NaOH solution needed...Ch. 9 - Prob. 9.98QPCh. 9 - Prob. 9.99QPCh. 9 - The concentration of Cu2+ ions in the water (which...Ch. 9 - How many grams of NaCl are required to precipitate...Ch. 9 - Prob. 9.102QPCh. 9 - Prob. 9.103QPCh. 9 - Prob. 9.104QPCh. 9 - Prob. 9.105QPCh. 9 - Which of the following best represents the...Ch. 9 - Prob. 9.107QPCh. 9 - Prob. 9.108QPCh. 9 - Prob. 9.109QPCh. 9 - Prob. 9.110QPCh. 9 - Prob. 9.111QPCh. 9 - A 5.00 102 mL sample of 2.00 M HCl solution is...Ch. 9 - Calculate the volume of a 0.156 M CuSO4 solution...Ch. 9 - Prob. 9.114QPCh. 9 - Prob. 9.115QPCh. 9 - Prob. 9.116QPCh. 9 - Prob. 9.117QPCh. 9 - Prob. 9.118QPCh. 9 - Prob. 9.119QPCh. 9 - Prob. 9.120QPCh. 9 - Prob. 9.121QPCh. 9 - Prob. 9.122QPCh. 9 - Prob. 9.123QPCh. 9 - Prob. 9.124QPCh. 9 - Classify the following reactions according to the...Ch. 9 - Prob. 9.126QPCh. 9 - Prob. 9.127QPCh. 9 - Prob. 9.128QPCh. 9 - Prob. 9.129QPCh. 9 - Prob. 9.130QPCh. 9 - Prob. 9.131QPCh. 9 - Prob. 9.132QPCh. 9 - Prob. 9.133QPCh. 9 - Prob. 9.134QPCh. 9 - Prob. 9.135QPCh. 9 - Prob. 9.136QPCh. 9 - The concentration of lead ions (Pb2+) in a sample...Ch. 9 - Prob. 9.138QPCh. 9 - Prob. 9.139QPCh. 9 - Prob. 9.140QPCh. 9 - Prob. 9.141QPCh. 9 - Prob. 9.142QPCh. 9 - Prob. 9.143QPCh. 9 - The following are common household compounds: salt...Ch. 9 - Prob. 9.145QPCh. 9 - A 0.8870-g sample of a mixture of NaCl and KCl is...Ch. 9 - Prob. 9.147QPCh. 9 - Prob. 9.148QPCh. 9 - Acetylsalicylic acid (HC9H7O4) is a monoprotic...Ch. 9 - Prob. 9.150QPCh. 9 - Prob. 9.151QPCh. 9 - Prob. 9.152QPCh. 9 - Prob. 9.153QPCh. 9 - Prob. 9.154QPCh. 9 - Prob. 9.155QPCh. 9 - Prob. 9.156QPCh. 9 - Prob. 9.157QPCh. 9 - Prob. 9.158QPCh. 9 - Prob. 9.159QPCh. 9 - Prob. 9.160QPCh. 9 - Prob. 9.161QPCh. 9 - Prob. 9.162QPCh. 9 - Give a chemical explanation for each of the...Ch. 9 - Prob. 9.164QPCh. 9 - The following cycle of copper experiment is...Ch. 9 - Use the periodic table framework given here to...Ch. 9 - A 22.02-mL solution containing 1.615 g Mg(NO3)2 is...Ch. 9 - Because the acid-base and precipitation reactions...Ch. 9 - Prob. 9.1KSPCh. 9 - Prob. 9.2KSPCh. 9 - Prob. 9.3KSPCh. 9 - Prob. 9.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- i need help with the followingarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO(g) +Cl₂ (g) = 2NOC1 (g) AGº = -41. kJ Now suppose a reaction vessel is filled with 8.90 atm of chlorine (C12) and 5.71 atm of nitrosyl chloride (NOC1) at 1075. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. atm ☑ 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0.29 mol of NaOH is added to 1.0 L of a 1.2M HCN solution. bases: ☑ other: 0.09 mol of HCl is added to acids: 1.0 L of a solution that is bases: 0.3M in both HCN and KCN. other: 0,0,... ? 00. 18 Ar 日arrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forwardHomework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forward
- Identifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY