
CHEMISTRY
2nd Edition
ISBN: 9781593995782
Author: OpenStax
Publisher: XANEDU PUBLISHING
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 82E
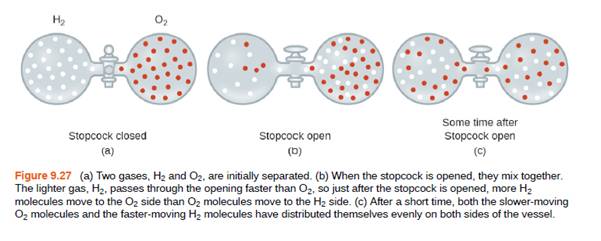
Explain why the numbers of molecules are not identical in the left- and tight-hand bulbs shown in the center illustration of Figure 9.27.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
PLEASE HELP! URGENT!
"Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this
fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation
and determine the enthalpy of this reaction:
CO(g) + O2(g) → CO₂(g) + 282.8 kJ
H2(g) + O2(g) → H₂O(g) + 241.8 kJ
MacBook Air
Page of 3
4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you
know?
NH3(g) + HCl(g) → NH4Cl(s)
AH=-176.0 kJ
AS-284.8 J-K-1
Chapter 9 Solutions
CHEMISTRY
Ch. 9 - Why are sharp knives more effective than dull...Ch. 9 - Why do some small bridges have weight limits that...Ch. 9 - Why should you roll or belly-crawl rather than...Ch. 9 - A typical barometric pressure in Redding....Ch. 9 - A typical barometric pressure in Denver, Colorado,...Ch. 9 - A typical barometric pressure in Kansas City is...Ch. 9 - Canadian tire pressure gauges are marked in units...Ch. 9 - Dining the Viking landings on Mars, the...Ch. 9 - The pressure of the atmosphere on the surface of...Ch. 9 - A medical laboratory catalog describes the...
Ch. 9 - Consider this scenario and answer the following...Ch. 9 - Why is it necessary to use a nonvolatile liquid in...Ch. 9 - The pressure of a sample of gas is measured at sea...Ch. 9 - The pressure of a sample of gas is measured with...Ch. 9 - The pressure of a sample of gas is measured at sea...Ch. 9 - The pressure of a sample of gas ¡s measured a sea...Ch. 9 - How would the use of a volatile liquid affect the...Ch. 9 - Sometimes leaving a bicycle in the sun on a hot...Ch. 9 - Explain how the volume of the bubbles exhausted by...Ch. 9 - One way to state Boyle’s law is All other things...Ch. 9 - An alternate way to state Avogadro’s law is A1l...Ch. 9 - How would the graph in Figure 9.12 change if the...Ch. 9 - How would the graph in Figure 9.13 change if the...Ch. 9 - In addition to the data found in Figure 9.13, what...Ch. 9 - Determine the volume of 1 mol of CH4 gas at 150 K...Ch. 9 - Determine the pressure of the gas in the syringe...Ch. 9 - A spray can is used until it is empty except for...Ch. 9 - What is the temperature of an 11.2-L sample of...Ch. 9 - À 2.50-L volume of hydrogen measured at —196 C is...Ch. 9 - A balloon inflated with three breaths of air has a...Ch. 9 - A weather balloon contains 8.80 moles of helium at...Ch. 9 - The volume of an automobile air bag was 66.8 L...Ch. 9 - How many moles of gaseous boron trifluoride, BF3,...Ch. 9 - Iodine, I2, is a solid at room temperature but...Ch. 9 - How many grams of gas are present in each of the...Ch. 9 - A high altitude balloon is filled with 1041104 L...Ch. 9 - A cylinder of medical oxygen has a volume of 3S.4...Ch. 9 - A large scuba tank (Figure 9.16) with a volume of...Ch. 9 - A 20.0-L cylinder containing 11.34 kg of butane,...Ch. 9 - While resting, the average 70-kg human male...Ch. 9 - For a given amount of gas showing ideal behavior,...Ch. 9 - A liter of methane gas, CH4, at STP contains more...Ch. 9 - The effect of chlorofluorocarbons (such as CCl2F2)...Ch. 9 - As 1 g of (lie radioactive element radium decays...Ch. 9 - A balloon that is 100.21 L at 21 C and 0.981 atm...Ch. 9 - If the temperature of a fixed amount of a gas is...Ch. 9 - If the volume of a fixed amount of a gas is...Ch. 9 - What is the density of laughing gas, dinitrogen...Ch. 9 - Calculate the density of Freon 12, CF2Cl2, at 30.0...Ch. 9 - Which is denser at the same temperature and...Ch. 9 - A cylinder of O2(g) used in breathing by emphysema...Ch. 9 - What is the molar mass of a gas if 0.0494 g of the...Ch. 9 - What is the molar mass of a gas if 0.281 g of the...Ch. 9 - How could you show experimentally that the...Ch. 9 - The density of a certain gaseous fluoride of...Ch. 9 - Consider this question: What is the molecular...Ch. 9 - A 36.0—L cylinder of a gas used for calibration of...Ch. 9 - A cylinder of a gas mixture used for calibration...Ch. 9 - A sample of gas isolated from unrefined petroleum...Ch. 9 - A mixture of 0.200 g of 1.00 g of and 0.820 g of...Ch. 9 - Most mixtures of hydrogen gas with oxygen gas are...Ch. 9 - A commercial mercury vapor analyzer can detect in...Ch. 9 - A sample of carbon monoxide was collected over...Ch. 9 - In an experiment in a general chemistry...Ch. 9 - Joseph Priestley first prepared pure oxygen by...Ch. 9 - Cavendish prepared hydrogen in 176G by the novel...Ch. 9 - The chlorofluorocarbon CCl2F2 can be recycled into...Ch. 9 - Automobile air bags are inflated with nitrogen...Ch. 9 - Lime, CaO, is produced by heating calcium...Ch. 9 - Before small batteries were available, carbide...Ch. 9 - Calculate the volume of oxygen required to burn...Ch. 9 - What volume of O2 at STP is required to oxidize...Ch. 9 - Consider the following questions: (a) What is the...Ch. 9 - Methanol, CH3OH, is produced industrially by the...Ch. 9 - What volume of oxygen a 423.0 K and a pressure of...Ch. 9 - A 230-L sample of a colorless gas at STP...Ch. 9 - Ethanol, C2H5OH, is produced industrially from...Ch. 9 - One molecule of hemoglobin will combine with four...Ch. 9 - A sample of a compound of xenon and fluorine was...Ch. 9 - One method of analyzing amino acids is the van...Ch. 9 - A balloon filled with helium gas is found to take...Ch. 9 - Explain why the numbers of molecules are not...Ch. 9 - Starting with the definition of rate of effusion...Ch. 9 - Heavy water, D2O (molar mass = 20.03 g mol-1). can...Ch. 9 - Which of the following gases diffuse more slowly...Ch. 9 - During the discussion of gaseous diffusion for...Ch. 9 - Calculate the relative rate of diffusion of 1H2...Ch. 9 - A gas of unknown identity diffuses at a rate of...Ch. 9 - When two cotton plugs. one moistened with ammonia...Ch. 9 - Using the postulates of the kinetic molecular...Ch. 9 - Can the speed of a given molecule in a gas double...Ch. 9 - Describe what happens o the average kinetic energy...Ch. 9 - The distribution of molecular velocities in a...Ch. 9 - What is the ratio of the average kinetic energy of...Ch. 9 - A 1-L sample of CO initially at STP is heated to...Ch. 9 - The root mean square speed of H2, molecules at 25...Ch. 9 - Answer the following questions: (a) Is the...Ch. 9 - Show that the ratio of the rate of diffusion of...Ch. 9 - Graphs showing the behavior of several different...Ch. 9 - Explain why the plot of PV for CO2 differs from...Ch. 9 - Under which of the following sets of conditions...Ch. 9 - Describe the factors responsible for the deviation...Ch. 9 - For which of the following gases should the...Ch. 9 - A 0.245-L flask contains 0.467 mol CO2 at 159 C....Ch. 9 - Answer the following questions: (a) If XX behaved...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
2. List the subdivisions of the thoracic and abdominopelvic cavities.
Human Anatomy & Physiology (2nd Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
4. Three groups of nonvascular plants are _______, ______, and _______. Three groups of seedless vascular plant...
Biology: Life on Earth (11th Edition)
Knowledge Booster
Similar questions
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning