
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357391594
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 80P
9-86 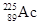 is effective in prostate cancer therapy when administered at kBq levels. If an antibody tagged with
is effective in prostate cancer therapy when administered at kBq levels. If an antibody tagged with 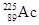 has an intensity of 2 million Bq/mg and if a solution contains 5 mg/L tagged antibody, how many milliliters of the solution should you use for an injection to administer 1 kBq intensity?
has an intensity of 2 million Bq/mg and if a solution contains 5 mg/L tagged antibody, how many milliliters of the solution should you use for an injection to administer 1 kBq intensity?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Vnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling
point, choose 2 next to the substance with the next highest boiling point, and so on.
substance
C
D
chemical symbol,
chemical formula
or Lewis structure.
CH,-N-CH,
CH,
H
H 10: H
C-C-H
H H H
Cale
H 10:
H-C-C-N-CH,
Bri
CH,
boiling point
(C)
Сен
(C) B
(Choose
Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!
Q1: Answer the questions for the reaction below:
..!! Br
OH
a) Predict the product(s) of the reaction.
b) Is the substrate optically active? Are the product(s) optically active as a mix?
c) Draw the curved arrow mechanism for the reaction.
d) What happens to the SN1 reaction rate in each of these instances:
1. Change the substrate to
Br
"CI
2. Change the substrate to
3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF
4. Increase the substrate concentration by 3-fold.
Chapter 9 Solutions
Introduction to General, Organic and Biochemistry
Ch. 9.3 - Problem 9-1 Iodine-139 is a beta emitter. Write an...Ch. 9.3 - Problem 9-2 Thorium-223 is an alpha emitter. Write...Ch. 9.3 - Prob. 9.3QCCh. 9.3 - Problem 9-4 Thallium-201, a radioisotope used to...Ch. 9.4 - Problem 9-5 Barium-122 has a half-life of 2...Ch. 9.5 - Problem 9-6 A radioactive isotope in a 9.0-mL vial...Ch. 9.5 - Prob. 9.7QCCh. 9 - What is the relationship between frequency and...Ch. 9 - How does energy relate to frequency and...Ch. 9 - Prob. 3P
Ch. 9 - 9-10 Microwaves are a form of electromagnetic...Ch. 9 - Prob. 5PCh. 9 - Prob. 6PCh. 9 - Prob. 7PCh. 9 - 9-14 Write the symbol for a nucleus with the...Ch. 9 - Prob. 9PCh. 9 - Prob. 10PCh. 9 - Prob. 11PCh. 9 - Prob. 12PCh. 9 - Prob. 13PCh. 9 - Prob. 14PCh. 9 - Prob. 15PCh. 9 - Prob. 16PCh. 9 - Prob. 17PCh. 9 - Prob. 18PCh. 9 - Prob. 19PCh. 9 - Prob. 20PCh. 9 - Prob. 21PCh. 9 - Prob. 22PCh. 9 - Prob. 23PCh. 9 - Prob. 24PCh. 9 - Prob. 25PCh. 9 - Prob. 26PCh. 9 - Prob. 27PCh. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - Prob. 32PCh. 9 - 9-39 If you work in a lab containing radioisotopes...Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Prob. 41PCh. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Prob. 49PCh. 9 - Prob. 50PCh. 9 - Prob. 51PCh. 9 - Prob. 52PCh. 9 - Prob. 53PCh. 9 - Prob. 54PCh. 9 - Prob. 55PCh. 9 - Prob. 56PCh. 9 - Prob. 57PCh. 9 - Prob. 58PCh. 9 - Prob. 59PCh. 9 - Prob. 60PCh. 9 - Prob. 61PCh. 9 - Prob. 62PCh. 9 - Prob. 63PCh. 9 - Prob. 64PCh. 9 - Prob. 65PCh. 9 - Prob. 66PCh. 9 - Prob. 67PCh. 9 - Prob. 68PCh. 9 - Prob. 69PCh. 9 - Prob. 70PCh. 9 - Prob. 71PCh. 9 - Prob. 72PCh. 9 - Prob. 73PCh. 9 - Prob. 74PCh. 9 - Prob. 75PCh. 9 - Prob. 76PCh. 9 - Prob. 77PCh. 9 - Prob. 78PCh. 9 - Prob. 79PCh. 9 - 9-86 is effective in prostate cancer therapy when...Ch. 9 - Prob. 81PCh. 9 - Prob. 82PCh. 9 - Prob. 83PCh. 9 - Prob. 84PCh. 9 - Prob. 85PCh. 9 - Prob. 86PCh. 9 - Prob. 87PCh. 9 - Prob. 88PCh. 9 - Prob. 89PCh. 9 - Prob. 90PCh. 9 - Prob. 91PCh. 9 - Prob. 92PCh. 9 - Prob. 93PCh. 9 - Prob. 94PCh. 9 - Prob. 95P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Experiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forward
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
- Q5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY