
EBK ESSENTIAL ORGANIC CHEMISTRY
3rd Edition
ISBN: 8220100659461
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 56P
When a
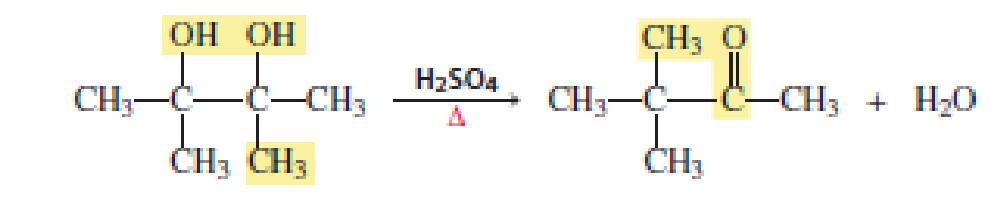
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lewis structure of a generic amino acid labeling atoms, bonds and lone pairs
If multiple stereoisomers are formed, be sure to draw all products using appropriate wedges and dashes.
This question has two parts. Be sure to complete both parts before submitting your answer.
Draw the actual, correct product for this reaction.
CH3
Br
NaOMe
MeOH
CH₂
Δ
H₂C
CH3
Incorrect
H
Draw the structures of the precursors to 2-methylbutanenitrile given the reagents below.
PBr3
Structure
A
NaCN
Structure
B
H3C
CN
CH3
Chapter 9 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
Ch. 9.1 - Draw the structures of straight-chain alcohols...Ch. 9.1 - Prob. 2PCh. 9.1 - Prob. 3PCh. 9.2 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 9.2 - Prob. 5PCh. 9.2 - The observed relative reactivities of primary,...Ch. 9.4 - Which of the following alcohols would dehydrate...Ch. 9.4 - Prob. 10PCh. 9.4 - Prob. 11PCh. 9.4 - Prob. 12P
Ch. 9.4 - Prob. 13PCh. 9.5 - What product will be obtained from the reaction of...Ch. 9.5 - Prob. 15PCh. 9.6 - a. What is each ethers systematic name? 1....Ch. 9.8 - Draw the structure of the following: a....Ch. 9.8 - Prob. 20PCh. 9.8 - Would you expect the reactivity of a five-membered...Ch. 9.9 - Explain why the two arene oxides in Problem 22...Ch. 9.9 - Which compound is more likely to be...Ch. 9.11 - The following three nitrogen mustards were studied...Ch. 9 - What are the common and systematic names of the...Ch. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - What is the major product obtained from the...Ch. 9 - Draw structures for the following: a....Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Ethylene oxide reacts readily with HO.because of...Ch. 9 - Propose a mechanism for each of the following...Ch. 9 - Which of the following ethers would be obtained in...Ch. 9 - Show how each of the following syntheses could be...Ch. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Propose a mechanism for each of the following...Ch. 9 - a. Propose a mechanism for the following reaction:...Ch. 9 - Three arene oxides can be obtained from...Ch. 9 - Prob. 48PCh. 9 - The following reaction takes place several times...Ch. 9 - Show how each of the following compounds could be...Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - What alkenes would you expect to be obtained from...Ch. 9 - Triethylenemelamine (TEM) is an antitumor agent....Ch. 9 - When a diol that has OH groups on adjacent carbons...Ch. 9 - What product is obtained when...Ch. 9 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3-Methylaniline with NaNO2 in HCl at 0°C and subsequent reaction with water in an acidic medium.arrow_forwardThe reaction of cyclohexanone with diethylamine.arrow_forwardYou're preparing the 2-1 parenteral nutrition that uses potassium phosphate vials (3 mmol of phosphate and 4.4 meq of potassium per mL) to add the phosphate to the parenteral nutrtion. How many meq of potassium are added to the parenteral nutrition when the phosphate is added? Potassium 65 meq Phosphate 24 mmol Sterile water qs ad 2000mlarrow_forward
- The combined calcium and phosphorus in a parenteeral nutrition should not exceed 45 mEq/L. if the parenteral nutrition order shown containes 2mEq PO4/mmol, what is the combined calcium and phosphorus (mEq/L) in the parenteral nutrition order? Parenteral nutrition order: Potassium 65 mEq, Phosphate 24 mmol, Calcium 12 mEqarrow_forwardpls help on all asked.arrow_forward29. The following reaction takes place. A2B(s) = 2A (aq) + B(aq) + heat. If 2.50 moles of A2B is added into a 2.00 L container, it is found that 25.0% of the reactant dissociates to reach equilibrium. Find the solubility product constant, Ksp, for this reaction. Note: Your answer must include an ICE table.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY