
Microbiology with Diseases by Body System (4th Edition)
4th Edition
ISBN: 9780321918550
Author: Robert W. Bauman Ph.D.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 1VI
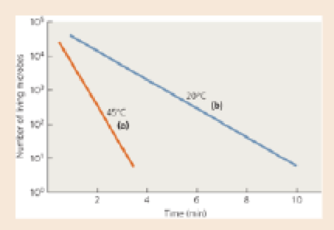
Calculate the decimal reduction time (D) for the two temperatures in the following graph.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sugar solution is being heated to 83 o C in a jacketed pan made from stainless steel, 1.6 mm thick. Heat is supplied by condensing steam at 200 kPa gauge in the jacket. Calculate the rate of heat transfer if the surface heat transfer coefficients for condensing steam and sugar solution are 12000 J/m2-s-oC and 3000 J/m2-s-oC, respectively. Thermal conductivity for the stainless steel is 21 J/m-s-oC and the surface area of the pan is 1.4 m 2 .
The temperature dependence of the vapor pressure is given by the equation:
ΔΗ
RT
Inp = In A –
Where:
p= vapor pressure
T = temperature
A = pre-exponential constant
AHvap = enthalpy of vaporization
In order to solve for the enthalpy of vaporization, AHvap, you must:
Step One
Add the same expression to each side of the equation to leave the term that includes the variable by itself on the right-hand side of the expression:
(Be sure that the answer field changes from light yellow to dark yellow before releasing your answer)
ΔΗ
RT
+ Inp =
+ In A
Drag and drop your selection from the following list to complete the answer:
1
1
In
In A
- In A
K
If you measured the rate of reaction at 20°C to be 1.11 x 10-5 M/s when using 0.080 M I1- and 0.040 M S2O82-. Approximately how long will the reaction take if you were to increase the temperature to 30 °C?
Chapter 9 Solutions
Microbiology with Diseases by Body System (4th Edition)
Ch. 9 - Why does milk eventually go bad despite being...Ch. 9 - Prob. 1EDCSCh. 9 - Why are BSL-4 suits pressurized? Why not just wear...Ch. 9 - Prob. 3TMWCh. 9 - Prob. 4TMWCh. 9 - Prob. 1MCCh. 9 - Prob. 2MCCh. 9 - Prob. 3MCCh. 9 - Prob. 4MCCh. 9 - Prob. 5MC
Ch. 9 - Prob. 6MCCh. 9 - Prob. 7MCCh. 9 - Prob. 8MCCh. 9 - The presarva1icn of beef jerky from microbial...Ch. 9 - Prob. 10MCCh. 9 - Which of the following substances would most...Ch. 9 - Which of the following adjectives best describes a...Ch. 9 - Prob. 13MCCh. 9 - Prob. 14MCCh. 9 - Prob. 15MCCh. 9 - Prob. 16MCCh. 9 - Which of the following disinfectants acts against...Ch. 9 - Which of the following disinfectants contains...Ch. 9 - Prob. 19MCCh. 9 - Prob. 20MCCh. 9 - Describe three types of microbes that are...Ch. 9 - Compare and contrast four tests that have been...Ch. 9 - Prob. 3SACh. 9 - Why do warm disinfectant chemicals generally work...Ch. 9 - Why are Gram-negative bacteria more susceptible to...Ch. 9 - Describe five physical methods of microbial...Ch. 9 - Prob. 7SACh. 9 - Prob. 8SACh. 9 - Compare and contrast desiccation and...Ch. 9 - Prob. 10SACh. 9 - Prob. 11SACh. 9 - Prob. 12SACh. 9 - Prob. 13SACh. 9 - What are some advantages and disadvantages of...Ch. 9 - Prob. 15SACh. 9 - Calculate the decimal reduction time (D) for the...Ch. 9 - Prob. 2VICh. 9 - Prob. 1CTCh. 9 - Prob. 2CTCh. 9 - Prob. 3CTCh. 9 - Prob. 4CTCh. 9 - Over 1000 people developed severe diarrhea, and at...Ch. 9 - Prob. 6CTCh. 9 - Prob. 7CTCh. 9 - Prob. 8CTCh. 9 - Prob. 9CTCh. 9 - Prob. 10CTCh. 9 - Prob. 11CTCh. 9 - Where should you place a sterilization indicator...Ch. 9 - Why is liquid water necessary for microbial...Ch. 9 - Prob. 14CTCh. 9 - During the fall 2001 bioterrorist attack in which...Ch. 9 - What common household antiseptic contains a heavy...Ch. 9 - What is the phenol coefficient of phenol when used...Ch. 9 - Prob. 1CM
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Combustion of a fuel sample in a bomb calorimeter increases the temperature of the entire system by 5.10 °C if the calorimeter contains 1700 g of water, but only by 4.00 °C if the calorimeter contains 2200 g of water. What is the heat capacity of the dry bomb calorimeter assembly? Assume that the specific heat capacity of water is 4.18 J g–1 °C–1.arrow_forwardHow is Kw defined, and what is its numerical value at 25 °C (298 K)?arrow_forwardAt what concentration of S(expressed as a multiple of KM) will νo=0.95Vmax?arrow_forward
- I need help pleasearrow_forwardFor the following reaction, 4.91 grams of water are mixed with excess chlorine gas. The reaction yields 12.5 grams of hydrochloric acid.chlorine (g) + water (l) hydrochloric acid (aq) + chloric acid (HClO3) (aq) What is the theoretical yield of hydrochloric acid ? grams What is the percent yield of hydrochloric acid ? %arrow_forwardA 100.0 mL sample of 0.200 M aqueous hydrochloric acid is added to 100.0 ml of 0.200 M aqueous ammonia in a calorimeter whose heat capacity (excluding any water) is 480.0 J/K. The following reaction occurs when the two solutions are mixed. HCl(aq)+NH,(aq)—NH_Cl(aq) The temperature increase is 2.34 °C. Calculate AH per mole of HCI and NH, reacted. Select one: о a. -1.96 KJ/mol b. 154 KJ/mol O c. 485 KJ/mol d. 1.96 KJ/mol Jm e. -154 J/molarrow_forward
- How many grams of potassium chlorate decompose to potassium chloride and 660. mL of O2 at 128. °C and 726. torr? Round your answer to 3 significant figures. 2KCIO3(s) 2KCI(s) + 302(g) Note: Reference the Conversion factors for non-SI units and Fundamental constants tables for additional information. g × Garrow_forwardFor the following results of Thermodynamics of Borax Solubility, the volume of Borax solution titrated by HCI is 8.00 mL. Table 1. Volumes of hydrochloric acid required to titrate a saturated borax solution at varying temperatures. The hydrochloric acid was a solution standardized at 0.2912 M. Borax Volume added (mL) Temp. (°C) 8.00 8.00 8.00 8.00 8.00 HCI Volume (mL) 50.5 33.75 40.7 27.02 30.0 17.95 20.2 13.43 10.3 8.55 Using Thermodynamic formula (R= 8.31 J/K•mol) and the above results, (e) AS° = (J/K⚫mol) Type your answer...arrow_forwardFor the following results of Thermodynamics of Borax Solubility, the volume of Borax solution titrated by HCI is 8.00 mL. Table 1. Volumes of hydrochloric acid required to titrate a saturated borax solution at varying temperatures. The hydrochloric acid was a solution standardized at 0.2912 M. Borax Volume added (mL) Temp. (°C) 8.00 8.00 8.00 8.00 8.00 HCI Volume (mL) 50.5 33.75 40.7 27.02 30.0 17.95 20.2 13.43 10.3 8.55 Using Thermodynamic formula (R= 8.31 J/K⚫mol) and the above results, (b) What is the y-intercept of the best fitting line for this data? Type your answer...arrow_forward
- Plot a line weaver-burk graph for Km=6.30mM and Vmax=360uM/min when an experiment has 5 tubes with concentrations of substrates of 1.0mM, 10mM, 50mM, and 100mM.arrow_forwardUsing the formula V₁D₁ = V₂D2, what volume of a 1/100 (10-2) dilution would you need to produce 100 mL of a 1/10000 (10-4) dilution? (Note: provide your answer in decimal format to three decimal places.)arrow_forwardThe following data give the constant dissociation, Ka (for acetic acid at various temperatures): T(°C) 0 Ка 1.657 x 10-5 10 1.729 x 10-5 15 1.745 x 10-5 25 1.753 × 10-5 kJ ΔΗ° = 1.53 mol Submit Previous Answers Part B Correct Plot Inka vs. 1/T and fit a line to the points. The slope will correspond to —▲H° / R. 1/T InKa 3.66 x 10-3-11.01 3.53 x 10-3-10.97 3.47 x 10-3-10.96 3.36 × 10-3-10.95 InKa = (−184 K) (±) — 10.3 = slope -184 K = AH° R AH° -184 K = 0.008314 kJ/(mol·K) AH 1.53 kJ/mol = Use these data, and the constant dissociation at 25 °C, to calculate AS° for acetic acid ionization. (InK Express your answer with the appropriate units. μÅ AS° = Value Units ? -AH° + ᎡᎢ. AS° Rarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning


Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Haematology - Red Blood Cell Life Cycle; Author: Armando Hasudungan;https://www.youtube.com/watch?v=cATQFej6oAc;License: Standard youtube license