
a)
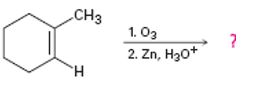
Interpretation:
The product of the reaction given showing both regiochemistry and stereochemistry is to be predicted.
Concept introduction:
Ozone adds to the double bond in
To give:
The product of the reaction given showing both regiochemistry and stereochemistry.
b)
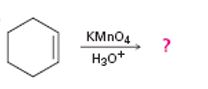
Interpretation:
The product of the reaction given showing both regiochemistry and stereochemistry is to be predicted.
Concept introduction:
Potassium permanganate in neutral or acidic solution cleaves alkenes to give carbonyl containing products. If hydrogens are present on the double bond
To predict:
The product of the reaction given showing both regiochemistry and stereochemistry.
c)
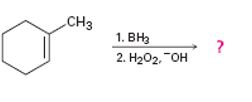
Interpretation:
The product of the reaction given showing both regiochemistry and stereochemistry is to be predicted.
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the less highly substituted carbon.
To predict:
The product of the reaction given showing both regiochemistry and stereochemistry.
d)
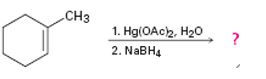
Interpretation:
The product of the reaction given showing both regiochemistry and stereochemistry is to be predicted.
Concept introduction:
In the oxymercuration-demercuration process, in the first step, the electrophilic addition of Hg2+ to the alkene gives a mercurinium ion. In the next step the mercurinium ion reacts with water to yield an organomercury product. In the last step, reaction with NaBH4 removes mercury to give a more highly substituted alcohol, corresponding to Markovnikov regiochemistry, as the product.
To predict:
The product of the reaction given showing both regiochemistry and stereochemistry
Trending nowThis is a popular solution!

Chapter 8 Solutions
ORGANIC CHEMISTRY W/OWL
- Draw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forwardWhat is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forward
- What are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardThe following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forwardThe chemical formula of "benzimidazole E" is C7H6N2. Draw it.arrow_forward
