
a)
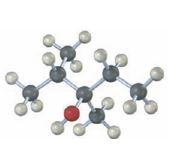
Interpretation:
The structures of
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with less number of alkyl substitutents.
The oxymercuration-demercuration reaction takes place with anti stereochemistry and results in Markovnokov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with more number of alkyl substitutents.
To give:
The structures of alkenes that would yield the alcohol shown on hydration.
To state:
Among hydroboration-oxidation and oxymercuration-demercuration methods which method can be used to prepare the alcohol from alkenes.
b)
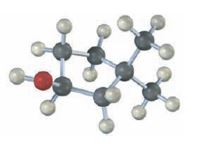
Interpretation:
The structures of alkenes that would yield the alcohol shown on hydration are to be given. Among hydroboration-oxidation and oxymercuration-demercuration, the method which can be used to prepare the alcohol also has to be stated.
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with less number of alkyl substitutents.
The oxymercuration-demercuration reaction takes place with anti stereochemistry and results in Markovnokov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with more number of alkyl substitutents.
To give:
The structures of alkenes that would yield the alcohol shown on hydration.
To state:
Among hydroboration-oxidation and oxymercuration-demercuration, the method which can be used to prepare the alcohol.
Trending nowThis is a popular solution!

Chapter 8 Solutions
ORGANIC CHEMISTRY W/OWL
- Which of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forwardCan I get help drawing my arrows #2arrow_forward
- Can I get some help with my arrows? I have included what the final outcome needs to look like. #3arrow_forwardPlease explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



