
Concept explainers
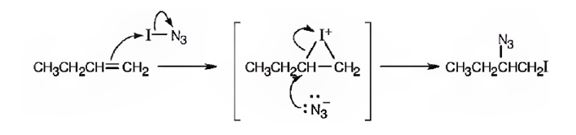
Given, iodine azide adds to 1- butane only one product shown results.
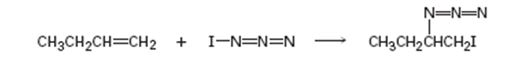
a) Add lone-pair electrons to the structure shown for IN3, and draw a second resonance form for the molecule.
Interpretation:
Lone pair of electrons is to be added to the structure of IN3 and another resonance form is to be drawn for it.
Concept introduction:
Lone pairs of electrons are those electrons which remain unshared on an atom in a molecule. Resonance forms differ only in the placements of their π or nonbonding electrons. Neither the position nor the hybridization of the atoms change in different resonance forms. Normal valence rules have to be followed.
To add:
Lone pair of electrons to the structure of IN3 and to draw another resonance form for it.
Answer to Problem 34MP
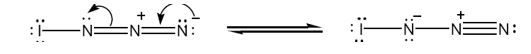
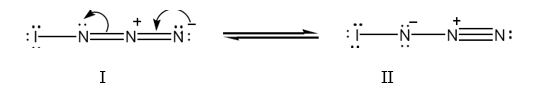
The structure of IN3 with lone pair of electrons added on each atom with another resonance form is shown below.
Explanation of Solution
Iodine has seven valence electrons (5s25p5) and nitrogen (2s22p3) has five (2s22p3) valence electrons. In the structure given, iodine has shared an electron with nitrogen in I-N bond. The other six electrons remain on it as lone pairs. Nitrogen is trivalent. The left nitrogen atom utilized three of its five electrons, one in bonding with iodine and other two in bonding with middle nitrogen. So it has a lone pair. The middle nitrogen has formed four bonds, two each with, left and right nitrogen. It has lost an additional electron to the nitrogen at right and has a positive charge. The nitrogen in the right, in addition to gaining an electron, has utilized only two of its five electrons for bonding with middle nitrogen. So it has a negative charge with two lone pair of electrons.
The structure of IN3 with lone pair of electrons added on each atom with another resonance form is shown below.

b) Calculate formal charges for the atoms in both resonance structures you drew for IN3 in part (a).
Interpretation:
The formal charges for the atoms in both resonance structures drawn for IN3 are to be calculated.
Concept introduction:
The formal charge on different atoms in a molecule can be calculated using the relation
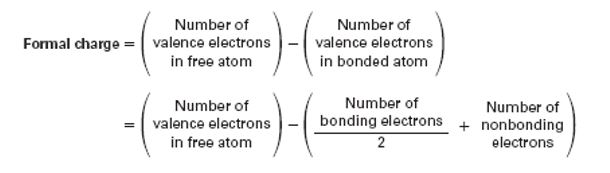
To calculate:
The formal charges for the atoms in both resonance structures drawn for IN3
Answer to Problem 34MP
Two resonance structures for IN3 with lone pair of electrons on each atom are shown below.
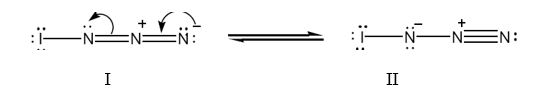
Formal charge for atoms in structure I:
Formal charge on iodine = 7-(2/2)-6 = 0
Formal charge on left nitrogen = 5-(6/2)-2 = 0
Formal charge on middle nitrogen = 5-(8/2)-0 = +1
Formal charge on right nitrogen = 5-(4/2)-4 = -1
Formal charge for atoms in structure II:
Formal charge on iodine = 7- (2/2)-6 = 0
Formal charge on left nitrogen = 5- (4/2)-4 = -1
Formal charge on middle nitrogen = 5-(8/2)-0 = +1
Formal charge on right nitrogen = 5-(6/2)-2 = 0
Explanation of Solution
The iodine atom has the outer electronic configuration 5s25p5. It has seven valence electrons. In structure I, the iodine atom has utilized one electron for forming a single bond with left nitrogen. It has six electrons as three lone pairs. Thus it has no formal charge.
Nitrogen has the outer electronic configuration 2s22p3. It has five valence electrons. It is trivalent. In structure I, the left nitrogen has used one electron for forming N-N bond and another electron for forming N-I bond. It has the remaining two electrons as a lone pair. Thus it has no formal charge.
The middle nitrogen has used four electrons two each in the two in N=N bonds. The middle nitrogen has lost one electron to the nitrogen in right and thus has a formal positive charge.
The right nitrogen has used two electrons for forming N=N bonds. It has gained one electron from middle nitrogen. Thus it has four electrons as two lone pairs. Thus it has a formal negative charge.
In structure II, the iodine atom has utilized one electron for forming a single bond with left nitrogen. It has six electrons as three lone pairs. Thus it has no formal charge.
Nitrogen has the outer electronic configuration 2s22p3. It has five valence electrons. It is trivalent. In structure II, the left nitrogen has used two electrons for forming N=N and an electron for forming N-I bond. It has four electrons as two lone pairs. Thus it has gained one electron and has a formal negative charge.
The middle nitrogen has used four electrons one for bonding with left nitrogen and three with right nitrogen. It has no lone pair of electrons. Thus it has lost one electron and has a formal positive charge.
The right nitrogen has used three electrons for forming three bonds with middle nitrogen. It has two electrons as a lone pair. Thus it has no a formal charge.
Two resonance structures for IN3 with lone pair of electrons on each atom are shown below.
Formal charge for atoms in structure I:
Formal charge on iodine = 7- (2/2)-6 = 0
Formal charge on left nitrogen = 5- (6/2)-2 = 0
Formal charge on middle nitrogen = 5-(8/2)-0 = +1
Formal charge on right nitrogen = 5-(4/2)-4 = -1
Formal charge for atoms in structure II:
Formal charge on iodine = 7- (2/2)-6 = 0
Formal charge on left nitrogen = 5- (4/2)-4 = -1
Formal charge on middle nitrogen = 5-(8/2)-0 = +1
Formal charge on right nitrogen = 5-(6/2)-2 = 0
c) In light of the result observed when IN3 adds to 1-butane, what is the polarity of the I-N3 bond? Propose a mechanism for the reaction using curved arrows to show the electron flow in each step.
Interpretation:
The polarity of I-N3 bond when it adds to 1-butene is to be stated. A mechanism is to be proposed for the reaction using curved arrows to show the electron flow in each step.
Concept introduction:
If the two atoms in a covalent bond differ in their electronegativity values, then the bond becomes polar with the less electronegative atom at the negative end and the more electronegative atom at the positive end of the dipole. The addition follows Markovnikov regiochemistry, the negative part gets added to the more alkyl substituted carbon in the double bond and the positive part gets added to the less alkyl substituted carbon in the double bond.
To state:
The polarity of I-N3 bond when it adds to 1-butene. To propose a mechanism for the reaction using curved arrows to show the electron flow in each step.
Answer to Problem 34MP
The polarity of I-N3 bond is
A mechanism for the reaction using curved arrows to show the electron flow in each step when I-N3 adds to 1-butene is shown below.
Explanation of Solution
In the product the iodine atom is attached to the less alkyl substituted carbon in double bond. If the addition occurs as per the Markovnikov regiochemistry, iodine must be the positive part in N-I3. The reaction is initiated by the attack of the π electrons of the double bond on the positively polarized iodine of N-I3 leading to the formation of an iodonium ion. In the second step, the N3- ion attacks the iodonium ion from the least hindered side to give the product.
The polarity of I-N3 bond is
A mechanism for the reaction of using curved arrows to show the electron flow in each step when I-N3 adds to 1-butene is shown below.

Want to see more full solutions like this?
Chapter 8 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Write the systematic name of each organic molecule: structure name × HO OH ☐ OH CI CI O CI OH OHarrow_forwardく Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forward
- theres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forward
- Organic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forward
- Draw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
