
a)

Interpretation:
The product of the reaction shown if the reaction was conducted in DMSO with water is to be given. The complete mechanism of the reaction including appropriate regiochemistry and stereochemistry is also to be given.
Concept introduction:
The addition of halogens to
To give:
The product of the reaction shown if the reaction was conducted in DMSO with water and to give the complete mechanism of the reaction including appropriate regiochemistry and stereochemistry.
b)
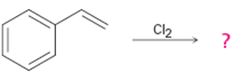
Interpretation:
The product of the reaction shown if the reaction was conducted in DMSO with water is to be given. The complete mechanism of the reaction including appropriate regiochemistry and stereochemistry is also to be given.
Concept introduction:
The addition of halogens to alkenes in the presence of aqueous DMSO results in the anti addition of the halohydrin, HOX, to the double bond. In the first step a cyclic halonium ion is formed by the attack of the double bond on the halogen. In the second step water attacks the halonium ion from the least shielded side to give an anti addition product. The addition obeys Markovnikov orientation. The negative part (OH) adds to the doubly bonded carbon atom which has more number of substituents.
To give:
The product of the reaction shown if the reaction was conducted in DMSO with water and to give the complete mechanism of the reaction including appropriate regiochemistry and stereochemistry.
c)
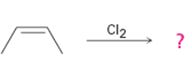
Interpretation:
The product of the reaction shown if the reaction was conducted in DMSO with water is to be given. The complete mechanism of the reaction including appropriate regiochemistry and stereochemistry is also to be given.
Concept introduction:
The addition of halogens to alkenes in the presence of aqueous DMSO results in the anti addition of the halohydrin, HOX, to the double bond. In the first step a cyclic halonium ion is formed by the attack of the double bond on halogen. In the second step water attacks the halonium ion from the least shielded side to give an anti addition product. The addition obeys Markovnokov orientation. The negative part (OH) adds to the doubly bonded carbon atom which has more number of substituents.
To give:
The product of the reaction shown if the reaction was conducted in DMSO with water and to give the complete mechanism of the reaction including appropriate regiochemistry and stereochemistry.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry - With Access (Custom)
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
