
ORG.CHEM.WILEYPLUSNEXTGEN.W/LLTEXT+STDY.
4th Edition
ISBN: 9781119832638
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 8.9, Problem 23ATS
Interpretation Introduction
Interpretation: The products of the catalytic hydrogenation of compound 1 with stereoisomers should be determined.
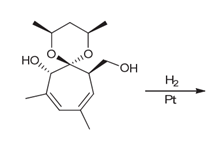
Concept introduction:
Some examples of addition reactions of
The catalytic hydrogenation of alkene leads to the formation of saturated hydrocarbons that are
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At the end of the silica gel production process, color changes occur during drying. Explain these color changes.
If CoCl2/H2O is dissolved in a mixture of H2O and concentrated HCl in a test tube, the tube is gently heated over a flame to approximately 80°C and then cooled externally. Explain the color changes that occur.
When producing silica gel, color changes occur at the end of the drying process. Explain these color changes.
Chapter 8 Solutions
ORG.CHEM.WILEYPLUSNEXTGEN.W/LLTEXT+STDY.
Ch. 8.3 - Provide a systematic name for each of the...Ch. 8.3 - Prob. 2CCCh. 8.3 - Prob. 3CCCh. 8.3 - Prob. 4CCCh. 8.5 - Prob. 5CCCh. 8.5 - Prob. 6CCCh. 8.5 - Prob. 1LTSCh. 8.5 - Prob. 7PTSCh. 8.5 - Prob. 8ATSCh. 8.5 - Prob. 9CC
Ch. 8.5 - Prob. 2LTSCh. 8.5 - Prob. 10PTSCh. 8.5 - Prob. 11ATSCh. 8.6 - Prob. 12CCCh. 8.6 - Prob. 13CCCh. 8.6 - Prob. 3LTSCh. 8.6 - Prob. 14PTSCh. 8.6 - Prob. 15ATSCh. 8.7 - Predict the product for each reaction, and predict...Ch. 8.7 - Prob. 17CCCh. 8.8 - Prob. 18CCCh. 8.8 - Prob. 19CCCh. 8.8 - Prob. 4LTSCh. 8.8 - Prob. 20PTSCh. 8.8 - Prob. 21ATSCh. 8.9 - Prob. 5LTSCh. 8.9 - Prob. 22PTSCh. 8.9 - Prob. 23ATSCh. 8.10 - Prob. 24CCCh. 8.10 - Prob. 6LTSCh. 8.10 - Prob. 25PTSCh. 8.10 - Prob. 26ATSCh. 8.10 - Prob. 27ATSCh. 8.11 - Prob. 7LTSCh. 8 - Prob. 47PP
Knowledge Booster
Similar questions
- Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in responsearrow_forwardCorrect each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forward
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
