
(a)
Interpretation:
Major and minor product should be drawn for the given substrates in E2 elimination reaction.
Concept introduction:
- Elimination reaction: In elimination reaction, two substituents are removed from the substrate to give the product in presence of base. Elimination reactions are two types, E1 and E2.
- E1 reaction: elimination follows stepwise mechanism.
- E2 reaction: elimination follows concerted pathway of mechanism.
- Elimination of compound in presence of bulky base leads to less substituted
alkene , in presence of strong base (not bulky) leads to more substituted alkene.
(b)
Interpretation:
Major and minor product should be drawn for the given substrates in E2 elimination reaction.
Concept introduction:
- Elimination reaction: In elimination reaction, two substituents are removed from the substrate to give the product in presence of base. Elimination reactions are two types, E1 and E2.
- E1 reaction: elimination follows stepwise mechanism.
- E2 reaction: elimination follows concerted pathway of mechanism.
- Elimination of compound in presence of bulky base leads to less substituted alkene, in presence of strong base (not bulky) leads to more substituted alkene.
(c)
Interpretation:
Major and minor product should be drawn for the given substrates in E2 elimination reaction.
Concept introduction:
- Elimination reaction: In elimination reaction, two substituents are removed from the substrate to give the product in presence of base. Elimination reactions are two types, E1 and E2.
- E1 reaction: elimination follows stepwise mechanism.
- E2 reaction: elimination follows concerted pathway of mechanism.
- Elimination of compound in presence of bulky base leads to less substituted alkene, in presence of strong base (not bulky) leads to more substituted alkene.
(d)
Interpretation:
Major and minor product should be drawn for the given substrates in E2 elimination reaction.
Concept introduction:
- Elimination reaction: In elimination reaction, two substituents are removed from the substrate to give the product in presence of base. Elimination reactions are two types, E1 and E2.
- E1 reaction: elimination follows stepwise mechanism.
- E2 reaction: elimination follows concerted pathway of mechanism.
- Elimination of compound in presence of bulky base leads to less substituted alkene, in presence of strong base (not bulky) leads to more substituted alkene.
Answer:
Explanation:
To find: the products for the given alkyl halide during E2 reaction.
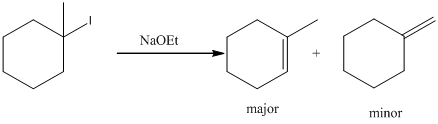
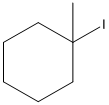
Given substrate is drawn below.
E2 elimination product for above substrate is drawn below.
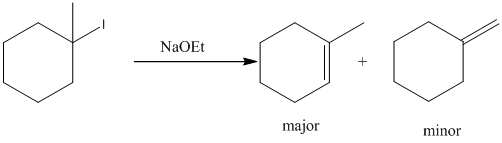
In the given elimination reaction used base is sodium ethoxide (NaoEt), which is strong base. Therefore, most substituted alkene is major product while the less substituted alkene is minor product.
(e)
Interpretation:
Major and minor product should be drawn for the given substrates in E2 elimination reaction.
Concept introduction:
- Elimination reaction: In elimination reaction, two substituents are removed from the substrate to give the product in presence of base. Elimination reactions are two types, E1 and E2.
- E1 reaction: elimination follows stepwise mechanism.
- E2 reaction: elimination follows concerted pathway of mechanism.
- Elimination of compound in presence of bulky base leads to less substituted alkene, in presence of strong base (not bulky) leads to more substituted alkene.
(f)
Interpretation:
Major and minor product should be drawn for the given substrates in E2 elimination reaction.
Concept introduction:
- Elimination reaction: In elimination reaction, two substituents are removed from the substrate to give the product in presence of base. Elimination reactions are two types, E1 and E2.
- E1 reaction: elimination follows stepwise mechanism.
- E2 reaction: elimination follows concerted pathway of mechanism.
- Elimination of compound in presence of bulky base leads to less substituted alkene, in presence of strong base (not bulky) leads to more substituted alkene.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry, Binder Ready Version
- please solve this, and help me know which boxes to check. Thank you so much in advance.arrow_forwardElectronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Describe how electronegativity is illustrated on the periodic table including trends between groups and periods and significance of atom size.arrow_forwardDefine the term “transition.” How does this definition apply to the transition metals?arrow_forward
- Describe how the properties of the different types of elements (metals, nonmetals, metalloids) differ.arrow_forwardUse a textbook or other valid source to research the physical and chemical properties of each element listed in Data Table 1 using the following as a guideline: Ductile (able to be deformed without losing toughness) and malleable (able to be hammered or pressed permanently out of shape without breaking or cracking) or not ductile or malleable Good, semi, or poor conductors of electricity and heat High or low melting and boiling points Occur or do not occur uncombined/freely in nature High, intermediate, or low reactivity Loses or gains electrons during reactions or is not reactivearrow_forwardProvide the Physical and Chemical Properties of Elements of the following elements listedarrow_forward
- Questions 4 and 5arrow_forwardFor a titration of 40.00 mL of 0.0500 M oxalic acid H2C2O4 with 0.1000 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin;2) 15 mL; 3) 20 mL; 4) 25 mL; 5) 40 mL; 6) 50 mL. Ka1 = 5.90×10^-2, Ka2 = 6.50×10^-5 for oxalic acid.arrow_forwardPredict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





