
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.5, Problem 12P
What
a.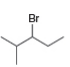 b.
b.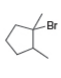 c.
c.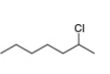 d.
d.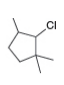
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
when 15.00 mL of 3.00 M NaOH was mixed in a caliorimeter with 13.50 mL of 3.00 M HCL, both initally at room temperature (22.00°C), the temperature increased 30.00°C. the resultant salt solution had a mass of 28.50g and a specific heat capacity of 3.74 J K^-1 g^-1. what is the heat capcity of the calorimeter in (J/ °C)? note: the molar enthalpy of neutralization per mole of HCl is -55.84kJ mol^-1
Don't used Ai solution and don't used hand raiting
Chapter 8 Solutions
ORGANIC CHEMISTRY
Ch. 8.1 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8.2 - Problem 8.2 Classify each alkene in the following...Ch. 8.2 - Prob. 3PCh. 8.2 - Prob. 4PCh. 8.2 - Problem 8.5 Label each pair of alkenes as...Ch. 8.2 - Problem 8.6 Which alkene in each pair is more...Ch. 8.2 - Problem 8.7 Several factors can affect alkene...Ch. 8.4 - Prob. 8PCh. 8.4 - Prob. 9PCh. 8.4 - Prob. 10P
Ch. 8.4 - Prob. 11PCh. 8.5 - Problem 8.12 What alkenes are formed from each...Ch. 8.6 - Prob. 13PCh. 8.6 - Problem 8.14 What alkenes are formed from each...Ch. 8.6 - Problem 8.15 How does each of the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 39PCh. 8 - Prob. 41PCh. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 56PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 63PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
Give the IUPAC name for each compound.
Organic Chemistry
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Check F1 三 www-awy.aleks.com/alekscgi/x/isl.exe/1o_u-igNslkr7j8P3JH-IvWymv180mkUcabkqJOgnjFoc724-61BXBxLvSRpvMeqRR- Homework 8 Chapter 17 & 18 Question 3 of 14 (1 point) | Question Attempt: 1 of Unlimited Draw the structures of the products formed by hydrolysis of the following tripeptide at physiological pH. Cys-Asn-Thr Note: Reference the Naturally occurring amino acids data table for additional information. Click and drag to start drawing a structure. 80 F3 F4 2 # 3 $ 4 45 % F5 9> F6 F7 27 W E R T Y U Sav © 2025 McGraw Hill LLC. All Rights Reserved * 8 DII F8 4 ( 9 F9arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardpls help kindly plsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY