
Interpretation:
Nucleophilic centers in the given compound have to be identified.
Concept Introduction:
Nucleophile is the one which are capable of donating a pair of electrons. The one which is capable of accepting a pair of electrons are known as electrophiles. The ions which possess a negative charge (lone pair of electrons) over it can act as a good nucleophile. Some of them are,
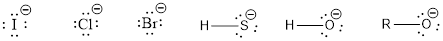
The compounds that contain lone pair of electrons also can act as nucleophile. Even though there is absence of negative charge, the presence of lone pair of electrons can make them to act as a good nucleophile. Some of the compounds are,
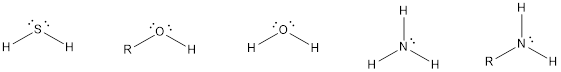
The compounds that contain unsaturated bonds also can act as nucleophile. This is because the unsaturated bonds are the regions where there is high electron density in space. Some of the compounds are,
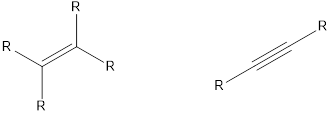
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
- Give the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forwardCalculate the residence time of strontium (Sr2+) in the world ocean, given that the average concentration of strontium in the world’s rivers is approximately 0.87 µmol L-1 (5 pts).arrow_forwardA package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forward
- How is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forwardPart II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forward
- Draw all formal charges on the structures below as is and draw 1 resonance structure that is more stable.arrow_forwardPart II. xiao isolated a compound TAD (Ca H 10 N₂) from tobacco and obtained its IR spectrum. Xiao proposed a chemical structure shown below: % Transmittance 4000 3500 3000 2500 2000 Wavenumber (cm-1) 1500 1000 (a) Explain why her proposed structure is inconsistent with the IR spectrum obtained (b) TAD exists as a tautomer of the structure xiao proposed. Draw the structure and explain why it is more compatible with the obtained spectrum. (C) what is the possible source for the fairly intense signal at 1621cm1arrow_forwardAE>AE₁ (Y/N) AE=AE₁ (Y/N) AEarrow_forwardTreatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. ? NH2 Br Br Propose a structural formula for compound A. You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. R3N C14H19NO2 + 2 R3NH*Br Aarrow_forwardCorrectly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





