![WebAssign for Zumdahl's Chemical Principles, 8th Edition [Instant Access], Single-Term](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
WebAssign for Zumdahl's Chemical Principles, 8th Edition [Instant Access], Single-Term
8th Edition
ISBN: 9780357119112
Author: Zumdahl; Steven S.
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 93E
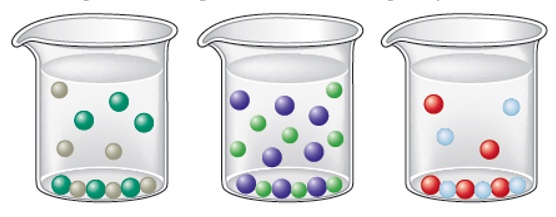
For which of the following is the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
QUESTION: Find the standard deviation for the 4 different groups
5.298
3.977
223.4
148.7
5.38
4.24
353.7
278.2
5.033
4.044
334.6
268.7
4.706
3.621
305.6
234.4
4.816
3.728
340.0
262.7
4.828
4.496
304.3
283.2
4.993
3.865
244.7
143.6
STDEV =
STDEV =
STDEV =
STDEV =
QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression'
*The images of the data showing 'coefficients for the standard curve' have been provided
Using the Nernst equation to calculate nonstandard cell voltage
Try Again
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
2+
2+
Sn²+ Ba(s)
(aq) + Ba (s) Sn (s) + Ba²+ (aq)
→>>
Suppose the cell is prepared with 6.10 M Sn
2+
2+
in one half-cell and 6.62 M Ba
in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
1.71 V
☐ x10
☑
5
0/5
?
00.
18
Ar
Chapter 8 Solutions
WebAssign for Zumdahl's Chemical Principles, 8th Edition [Instant Access], Single-Term
Ch. 8 - Prob. 1DQCh. 8 - Prob. 2DQCh. 8 - Mixing together solutions of acetic acid and...Ch. 8 - Sketch two pH curves, one for the titration of a...Ch. 8 - Sketch a pH curve for the titration of a weak acid...Ch. 8 - You have a solution of the weak acid HA and add...Ch. 8 - You have a solution of the weak acid HA and add...Ch. 8 - Prob. 8DQCh. 8 - You are browsing through the Handbook of...Ch. 8 - A friend tells you: "The constant Ksp of a salt is...
Ch. 8 - What happens to the Ksp value of a solid as the...Ch. 8 - Which is more likely to dissolve in an acidic...Ch. 8 - Prob. 13DQCh. 8 - Under what circumstances can the relative...Ch. 8 - Define a buffered solution. What makes up a...Ch. 8 - A good buffer generally contains relatively equal...Ch. 8 - How many of the following are buffered solutions?...Ch. 8 - Which of the following can be classified as buffer...Ch. 8 - Prob. 19ECh. 8 - Derive an equation analogous to the Henderson—...Ch. 8 - Calculate the pH of each of the following...Ch. 8 - Calculate the pH after 0.020 mole of HCl is added...Ch. 8 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 8 - The results of Exercises 21-23 illustrate an...Ch. 8 - One of the most challenging parts of solving...Ch. 8 - a. Calculate the pH of a buffered solution that is...Ch. 8 - Calculate the pH of a solution that is...Ch. 8 - Calculate the pH of a solution that is...Ch. 8 - Calculate the pH after 0.10mole of NaOH is added...Ch. 8 - Calculate the pH after 0.020mole of NaOH is added...Ch. 8 - Calculate the pH of a solution that is 0.40M H 2...Ch. 8 - Calculate the pH of a solution that is...Ch. 8 - Calculate the pH of a buffered solution prepared...Ch. 8 - A buffered solution is made by adding...Ch. 8 - Prob. 35ECh. 8 - How many moles of NaOH must be added to...Ch. 8 - Calculate the number of moles of HCl(g) that must...Ch. 8 - You make 1.00L of a buffered solution (pH=4.00) by...Ch. 8 - Calculate the mass of sodium acetate that must be...Ch. 8 - Calculate the pH after 0.010mole of gaseous HCl is...Ch. 8 - An aqueous solution contains dissolved...Ch. 8 - What volumes of 0.50MHNO2and0.50MNaNO2 must be...Ch. 8 - Phosphate buffers are important in regulating the...Ch. 8 - Carbonate buffers are important in regulating the...Ch. 8 - When a person exercises, muscle contractions...Ch. 8 - Which of the following mixtures would result in a...Ch. 8 - Which of the following mixtures would result in a...Ch. 8 - Calculate the pH of a solution formed by mixing...Ch. 8 - Consider the acids in Table 7.2. Which acid would...Ch. 8 - Consider the bases in Table 7.3. Which base would...Ch. 8 - A solution contains 1.0106MHOCl and an unknown...Ch. 8 - In Section 8.3 an equation was derived for the...Ch. 8 - Consider a weak acid HA with a Ka value of 1.6107....Ch. 8 - Consider the following pH curves for 100.0mL of...Ch. 8 - An acid is titrated with NaOH. The following...Ch. 8 - Consider the titration of a generic weak acid HA...Ch. 8 - Sketch the titration curve for the titration of a...Ch. 8 - Draw the general titration curve for a strong acid...Ch. 8 - Consider the following four titrations:...Ch. 8 - A student titrates an unknown weak acid HA to a...Ch. 8 - The following plot shows the pH curves for the...Ch. 8 - The figure in the preceding exercise shows the pH...Ch. 8 - Consider the titration of...Ch. 8 - Prob. 64ECh. 8 - Prob. 65ECh. 8 - Prob. 66ECh. 8 - Prob. 67ECh. 8 - Prob. 68ECh. 8 - Prob. 69ECh. 8 - Prob. 70ECh. 8 - Calculate the pH at the halfway point and at the...Ch. 8 - You have 75.0mLof0.10MHA. After adding...Ch. 8 - A student dissolves 0.0100mole of an unknown weak...Ch. 8 - What is an acid—base indicator? Define the...Ch. 8 - Two drops of indicator HIn(Ka=1.0109), where HIn...Ch. 8 - A certain indicator HIn has a pKa of 3.00 and a...Ch. 8 - Estimate the pH of a solution in which bromcresol...Ch. 8 - A solution has a pHof7.0. What would be the color...Ch. 8 - Which of the indicators in Fig. 8.8 could be used...Ch. 8 - Which of the indicators in Fig. 8.8 could be used...Ch. 8 - Which of the indicators in Fig. 8.8 could be used...Ch. 8 - Which of the indicators in Fig. 8.8 could be used...Ch. 8 - Methyl red has the following structure: It...Ch. 8 - Indicators can be used to estimate the pH values...Ch. 8 - When a diprotic acid, H2A, is titrated with NaOH,...Ch. 8 - A student was given a 0.10M solution of an unknown...Ch. 8 - Prob. 87ECh. 8 - Consider 100.0mLofa0.100M solution of...Ch. 8 - A 0.200-g sample of a triprotic acid...Ch. 8 - Consider the titration of 100.0mLof0.100MH3A...Ch. 8 - The titration of Na2CO3 with HCl has the following...Ch. 8 - Consider 100.0 mL of a solution of 0.200MNa2A,...Ch. 8 - For which of the following is the Ksp value of the...Ch. 8 - Ag2S(s) has a larger molar solubility than CuS...Ch. 8 - When Na3PO4(aq) is added to a solution containing...Ch. 8 - The common ion effect for ionic solids (salts) is...Ch. 8 - Prob. 97ECh. 8 - Calculate the solubility of each of the following...Ch. 8 - Use the following data to calculate the Ksp value...Ch. 8 - The concentration of Pb2+ in a solution saturated...Ch. 8 - The concentration of Ag+ in a solution saturated...Ch. 8 - The solubility of the ionic compound M2X3, having...Ch. 8 - For each of the following pairs of solids,...Ch. 8 - The solubility rules outlined in Chapter 4 say...Ch. 8 - Calculate the molar solubility of...Ch. 8 - The Ksp for silver sulfate (Ag2SO4) is 1.2105....Ch. 8 - Calculate the solubility (inmol/L) of Fe(OH)3...Ch. 8 - Prob. 108ECh. 8 - Calculate the solubility of solid Ca3(...Ch. 8 - The solubility of Ce( IO3)3 in a 0.20MKIO3...Ch. 8 - What mass of ZnS(Ksp=2.51022) will dissolve in...Ch. 8 - The concentration of Mg2+ in seawater is 0.052M....Ch. 8 - For the substances in Exercises 97and98, which...Ch. 8 - Explain the following phenomenon: You have a test...Ch. 8 - For which salt in each of the following groups...Ch. 8 - A solution is prepared by mixing 75.0mL of...Ch. 8 - Calculate the final concentrations of...Ch. 8 - A solution is prepared by mixing 50.0mLof0.10M Pb(...Ch. 8 - The Ksp of Al(OH)3 is 21032. At what pH will a...Ch. 8 - A solution is 1104M in NaF,Na2S, and Na3PO4. What...Ch. 8 - A solution contains 1.0105MNa3PO4. What is the...Ch. 8 - A solution contains 0.25MNi( NO3)2 and 0.25MCu(...Ch. 8 - Describe how you could separate the ions in each...Ch. 8 - If a solution contains either Pb2+(aq)orAg+(aq),...Ch. 8 - Sulfide precipitates are generally grouped as...Ch. 8 - Nanotechnology has become an important field, with...Ch. 8 - Prob. 127ECh. 8 - As a sodium chloride solution is added to a...Ch. 8 - The overall formation constant for HgI42is1.01030....Ch. 8 - A solution is prepared by adding 0.090mole of...Ch. 8 - Prob. 131ECh. 8 - Kf for the complex ion Ag( NH3)2+is1.7107. Ksp for...Ch. 8 - a. Using the Ksp for Cu(OH)2(1.61019) and the...Ch. 8 - The copper(I) ion forms a chloride salt that has...Ch. 8 - Solutions of sodium thiosulfate are used to...Ch. 8 - a. Calculate the molar solubility of AgI in pure...Ch. 8 - A series of chemicals was added to some...Ch. 8 - Will a precipitate of Cd(OH)2 form if 1.0mLof1.0M...Ch. 8 - Tris(hydroxymethyl)aminomethane, commonly called...Ch. 8 - Amino acids are the building blocks for all...Ch. 8 - The solubility of copper(II) hydroxide in water...Ch. 8 - The salts in Table 8.5, with the possible...Ch. 8 - You have the following reagents on hand: What...Ch. 8 - Prob. 144AECh. 8 - One method for determining the purity of aspirin...Ch. 8 - Another way to treat data from a pH titration is...Ch. 8 - Potassium hydrogen phthalate, known as KHP...Ch. 8 - sample of the ionic compound NaA, where A is the...Ch. 8 - What mass of Ca( NO3)2 must be added to 1.0L of a...Ch. 8 - The equilibrium constant for the following...Ch. 8 - Calculate the concentration of Pb2+ in each of the...Ch. 8 - Consider saturated solutions of the following...Ch. 8 - A certain acetic acid solution has pH=2.68 ....Ch. 8 - Calculate the volume of 1.5010-2MNaOH that must be...Ch. 8 - A 0.400M solution of ammonia was titrated with...Ch. 8 - A student intends to titrate a solution of a weak...Ch. 8 - The active ingredient in aspirin is...Ch. 8 - A solution is formed by mixing 50.0mL of 10.0MNaX...Ch. 8 - When phosphoric acid is titrated with a NaOH...Ch. 8 - Consider the following two acids: In two separate...Ch. 8 - Consider 1.0L of a solution that is 0.85MHOC6H5...Ch. 8 - What concentration of NH4Cl is necessary to buffer...Ch. 8 - Consider the following acids and bases:...Ch. 8 - Consider a buffered solution containing CH3NH3Cl...Ch. 8 - Consider the titration of 150.0mL of 0.100MHI by...Ch. 8 - Prob. 166AECh. 8 - Prob. 167AECh. 8 - Prob. 168AECh. 8 - Assuming that the solubility of Ca3( PO4)2(s) is...Ch. 8 - Order the following solids (ad) from least soluble...Ch. 8 - The Ksp for PbI2(s) is 1.410-8 . Calculate the...Ch. 8 - Prob. 172AECh. 8 - A 50.0-mL sample of 0.0413MAgNO3(aq) is added to...Ch. 8 - The Hg2+ ion forms complex ions with I as follows:...Ch. 8 - A buffer is made using 45.0mL of...Ch. 8 - What volume of 0.0100MNaOH must be added to 1.00L...Ch. 8 - For solutions containing salts of the form NH4X ,...Ch. 8 - Prob. 178CPCh. 8 - The copper(I) ion forms a complex ion with CN...Ch. 8 - Calcium oxalate (CaC2O4) is relatively insoluble...Ch. 8 - a. Calculate the molar solubility of SrF2 in...Ch. 8 - What is the maximum possible concentration of Ni2+...Ch. 8 - Prob. 183CPCh. 8 - Consider 1.0L of an aqueous solution that contains...Ch. 8 - Calculate the solubility of AgCN(s)(Ksp=2.21012)...Ch. 8 - Consider the titration of 100.0mL of a 1.00104M...Ch. 8 - Consider a solution formed by mixing 200.0mL of...Ch. 8 - Prob. 188CPCh. 8 - Calculate the pH of a solution prepared by mixing...Ch. 8 - Consider the titration of 100.0mL of 0.10M...Ch. 8 - In the titration of 100.0mL of a 0.0500M solution...Ch. 8 - Consider the titration curve in Exercise91 for the...Ch. 8 - Consider a solution prepared by mixing the...Ch. 8 - Prob. 194MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forward
- The following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forward
- Briefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY