
Concept explainers
(a)
Interpretation:
The Lewis structure of oleum has to be drawn.
Concept Introduction:
Lewis Structure: A Lewis structure shows a covalent bond as pair of electrons shared between two atoms.
Procedure to write Lewis formulas:
- 1) The symbols of the atoms that are bonded together in the molecule next to one another are arranged.
- 2) The total number of valence electrons in the molecule is calculated by adding the number of valence electrons for all the atoms in the molecules. If the species is an ion, then the charge of ion into account by adding electrons, if it is a negative ion or subtracting electrons if it is a positive ion.
- 3) A two-electron covalent bond is represented by placing a line between the atoms, which are assumed to be bonded to each other.
- 4) The remaining valence electrons as lone pairs about each atom are arranged so that the octet rule is satisfied for each other.
(a)
Answer to Problem 8H.8E
The Lewis structure of oleum is,
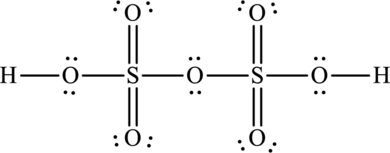
Explanation of Solution
Oleum is otherwise known as pyrosulphuric acid and its formula is
The number of valence electrons in hydrogen=
The number of valence electrons in oxygen=
The number of valence electrons in sulphur=
The total number of valence electrons in oleum is fifty-six.
The Lewis structure of oleum is,
(b)
Interpretation:
The formal charge on sulphur and oxygen in oleum has to be determined.
Concept Introduction:
Formal charge (F.C): The charges that assigned to each atom in a molecule or ion by a set of arbitrary rules and don not actually represent the actual charges on the atoms are called as formal charges.
The formal charge is calculated using the formula,
The Lewis structure with zero formal charge or least separated formal charges is the preferred structure of the molecule.
(b)
Answer to Problem 8H.8E
The formal charge in sulphur is zero.
The formal charge in oxygen is zero.
Explanation of Solution
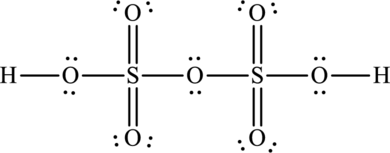
Oleum is otherwise known as pyrosulphuric acid and its formula is
The number of valence electrons in hydrogen=
The number of valence electrons in oxygen=
The number of valence electrons in sulphur=
The total number of valence electrons in oleum is fifty-six.
The Lewis structure of oleum is,
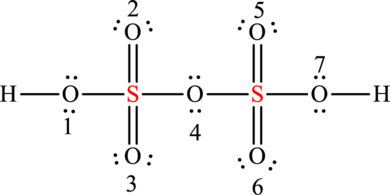
The formal charges on sulphur is,
The formal charge in sulphur is zero.
The formal charges on oxygen
The formal charge on oxygen
The formal charge on oxygen
The formal charge on oxygen
(c)
Interpretation:
The oxidation number of sulphur in oleum has to be drawn.
(c)
Answer to Problem 8H.8E
The oxidation number of sulphur in oleum is
Explanation of Solution
Oleum is otherwise known as pyrosulphuric acid and its formula is
The oxidation number of sulphur in
Want to see more full solutions like this?
Chapter 8 Solutions
CHEMICAL PRINCIPLES PKG W/SAPLING
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
