
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
8th Edition
ISBN: 9780135204634
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.9P
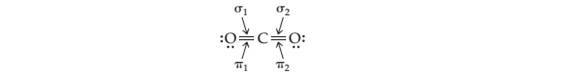
Which orbitals overlap to form the sigma and pi bonds in the following structure of carbon dioxide?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.
in the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0
calculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 M
Chapter 8 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
Ch. 8 - Prob. 8.1PCh. 8 - What is the number and geometric arrangement of...Ch. 8 - PRACTICE 8.3 Acetic acid, CH3CO2H , is the main...Ch. 8 - APPLY 8.4 Benzene, C6H6 , is a cyclic molecule in...Ch. 8 - PRACTICE 8.5 Identify the orbitals that overlap to...Ch. 8 - APPLY 8.6 Describe the bonding in propane, C3H8 ,...Ch. 8 - PRACTICE 8.7 Describe the hybridization of the...Ch. 8 - Describe the hybridization of each carbon atom in...Ch. 8 - Which orbitals overlap to form the sigma and pi...Ch. 8 - APPLY 8.10 Describe the hybridization of the...
Ch. 8 - Prob. 8.11PCh. 8 - Conceptual APPLY 8.12 Match the following...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14ACh. 8 - Prob. 8.15PCh. 8 - Prob. 8.16ACh. 8 - Prob. 8.17ACh. 8 - Prob. 8.18ACh. 8 - The B2 molecule has a MO diagram similar to that...Ch. 8 - Prob. 8.20ACh. 8 - PRACTICE 8.23 Draw two resonance structures for...Ch. 8 - APPLY 8.24 Draw two resonance structures for the...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - Caffeine is the most widely used stimulant...Ch. 8 - Prob. 8.26PCh. 8 - What is the geometry around the central atom in...Ch. 8 - What is the geometry around the central atom in...Ch. 8 - Three of the following molecular models have a...Ch. 8 - Identify each of the following sets of hybrid...Ch. 8 - The VSEPR model is a simple predictive tool that...Ch. 8 - The following ball-and-stick molecular model is a...Ch. 8 - The following ball-and-stick molecular model is a...Ch. 8 - Prob. 8.34CPCh. 8 - The dipole moment of methanol is =1.70D . Use...Ch. 8 - Methylarnine, CH3NH2 , is responsible for the odor...Ch. 8 - Prob. 8.37CPCh. 8 - Prob. 8.38SPCh. 8 - What shape do you expect for molecules that meet...Ch. 8 - How many charge clouds are there around the...Ch. 8 - Prob. 8.41SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - What shape do you expect for each of the following...Ch. 8 - What shape do you expect for each of the following...Ch. 8 - Prob. 8.45SPCh. 8 - Prob. 8.46SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - What bond angles do you expect for each of the...Ch. 8 - What bond angles do you expect for each of the...Ch. 8 - Acrylonitrile is used as the starting material for...Ch. 8 - Predict values for all bond angles in dimethyl...Ch. 8 - Oceanographers study the mixing of water masses by...Ch. 8 - A potential replacement for the chlorofluorocarbon...Ch. 8 - Explain why cyclohexane, a substance that contains...Ch. 8 - Like cyclohexane (Problem 8.54), benzene also...Ch. 8 - Use VSEPR theory to answer the following...Ch. 8 - Draw an electron-dot structure for each of the...Ch. 8 - What is the difference in spatial distribution...Ch. 8 - The average CC bond dissociation energy (D) is 350...Ch. 8 - What hybridization do you expect for atoms that...Ch. 8 - What spatial arrangement of charge clouds...Ch. 8 - What hybridization would you expect for the...Ch. 8 - What hybridization would you expect for the...Ch. 8 - Oxaloacetic acid is an intermediate involved in...Ch. 8 - The atoms in the amino acid glycine are connected...Ch. 8 - Describe the hybridization of the carbon atom in...Ch. 8 - Describe the hybridization of each carbon atom in...Ch. 8 - Bupropion, marketed as Wellbutr in, is a heavily...Ch. 8 - Efavirenz, marketed as Sustiva, is a medication...Ch. 8 - What is the hybridization of the B and N atoms in...Ch. 8 - Prob. 8.71SPCh. 8 - Aspirin has the following connections among atoms....Ch. 8 - The cation [HCNXeF]+ is entirely linear. Draw an...Ch. 8 - Acrylonitrile (C3H3N) is a molecule that is...Ch. 8 - The odor of cinnamon oil is due to cinnamaldehyde,...Ch. 8 - The following molecular model is a representation...Ch. 8 - Prob. 8.77SPCh. 8 - Which of the following substances would you expect...Ch. 8 - Which of the following substances would you expect...Ch. 8 - Why is the dipole moment of SO2 1.63 D hut that of...Ch. 8 - Prob. 8.81SPCh. 8 - The class of ions PtX42 , where X is a halogen,...Ch. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - Prob. 8.86SPCh. 8 - Prob. 8.87SPCh. 8 - What are the most important kinds of...Ch. 8 - Of the substances Xe, CH3Cl , and HF which has:...Ch. 8 - Methanol (CH3OH;bp=65C) boils nearly 230 °C higher...Ch. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - A liquid sample contains methylamine (CH3NH2)...Ch. 8 - Prob. 8.95SPCh. 8 - What is the difference in spatial distribution...Ch. 8 - Prob. 8.97SPCh. 8 - Use the MO energy diagram in Figure 8.22b to...Ch. 8 - Use the MO energy diagram in Figure 8.22 a to...Ch. 8 - The C2 molecule can be represented by an MO...Ch. 8 - Prob. 8.101SPCh. 8 - Prob. 8.102SPCh. 8 - Prob. 8.103SPCh. 8 - Draw a molecular orbital energy diagram for Li2 ....Ch. 8 - Calcium carbide, CaC2 , reacts with water to...Ch. 8 - At high temperatures, sulfur vapor is...Ch. 8 - Carbon monoxide is produced by incomplete...Ch. 8 - Make a sketch showing the location and geometry of...Ch. 8 - Make a sketch showing the location and geometry of...Ch. 8 - Prob. 8.110MPCh. 8 - Prob. 8.111MPCh. 8 - Prob. 8.112MPCh. 8 - Prob. 8.113MPCh. 8 - Just as individual bonds in a molecule are often...Ch. 8 - Cyclooctatetraenedian ion, C8H82 , is an organic...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
- find the pH of a buffer made from 0.20 M HNO2 and 0.10 M NaNO2. Ka= 4.0 x 10-4a) 4.00b) 3.40c) 3.70d) 3.10arrow_forwardthe Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.15 M HPO42-.a) 6.98b) 7.42c) 7.00d) 7.20arrow_forwardFind the equilibrium concentration of H3O+ starting with 0.072 M solution of acetic acid. Ka = 1.8 x 10-5. Acetic acid is HC2H3O2 (aq).HC2H3O2 (aq) + H2O (l) ⇌ H3O (aq) + C2H3O2- (aq) a) 1.3 x 10-6 b) 1.1 x 10-3 c) 1.5 x 10-2 d) 3.6 x 10-5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY