
To write:
The complete and balanced
a) 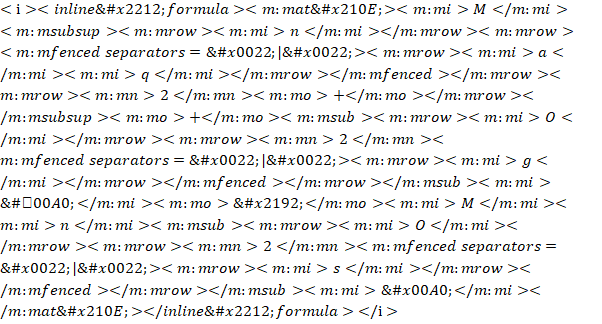 (basic solution)
(basic solution)
b)  (acidic solution)
(acidic solution)
c)
Answer to Problem 8.96QA
Solution:
Complete and balanced redox reaction is
a)
b)
c)
Explanation of Solution
1) Concept:
To balance a redox reaction, first we have to assign oxidation numbers to each of the elements in the compound and decide which element is going to be reduced and oxidized. Oxidation is loss of electrons or increase in oxidation number while reduction is gain of electrons or decrease in oxidation number.
2) Given:
i)
ii)  (acidic solution)
(acidic solution)
iii)
3) Calculations:
a)
i) The following preliminary expression relates the known reactant and products:
There are equal number of
ii) We perform an
As
iii) To balance these
iv) The ionic charge on the reactant is
v) Taking an inventory of atoms on both sides of the reaction arrow, we find that the left side has four more
Now the reaction is balanced.
b)
i) The following preliminary expression relates the known reactant and products:
There are equal numbers of  on both product and reactant sides, but there are unequal numbers of
on both product and reactant sides, but there are unequal numbers of
ii) We perform an
The
The change in the oxidation number is the same for oxidation and reduction.
iii) The ionic charge on the reactant is
iv) Taking an inventory of atoms on both sides of the reaction arrow, we find that the left side has four more
Now the reaction is balanced
c)
i) The following preliminary expression relates the known reactant and products:
There are unequal numbers of
ii) We perform an
The
The
The change in the oxidation number is the same for oxidation and reduction.
Also there are same number of charges on the reactant and product sides, so the reaction is balanced.
Conclusion:
In a redox reaction, substances either gain electrons or thereby undergo reduction, or they lose electrons and undergo oxidation. The given reaction is redox as the oxidation number of elements in the reactants changes during the reaction.
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry: An Atoms-Focused Approach
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





