
Concept explainers
(a)
Interpretation:
The apparatus has to be redrawn if the temperature is increased from
Concept introduction:
Charles’s law: States that volume is directly proportional to temperature when the gas is held at constant pressure and number of molecules.
(a)
Answer to Problem 8.26UKC
According to Charles’s law, if temperature increases at constant pressure, then volume also increases. Therefore, the apparatus is redrawn as given below:
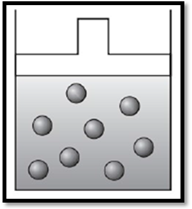
Explanation of Solution
Given data is that the temperature increases from
Charles’s law is the
From Charles’s law we know that
According to Charles’s law, if temperature increases at constant pressure, then volume also increases. Therefore, the apparatus is redrawn as given below:
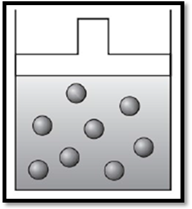
(b)
Interpretation:
The apparatus has to be redrawn if the pressure is increased from
Concept introduction:
Boyle’s law:
At fixed temperature, the volume of a fixed amount of gas is inversely proportional to the pressure exerted by the gas.
(b)
Answer to Problem 8.26UKC
According to Boyle’s law, if pressure increases at constant temperature, then volume decreases. Therefore, the apparatus is redrawn as given below:
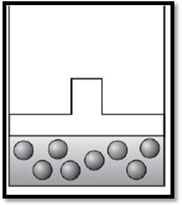
Explanation of Solution
Given data is that the pressure is increased from
Boyle’s law is the law which relates pressure and volume at a constant temperature and number of molecules.
From Boyle’s law:
According to Boyle’s law, if pressure increases at constant temperature, then volume decreases. Therefore, the apparatus is redrawn as given below:
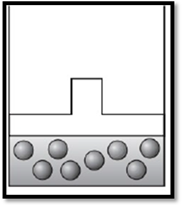
(c)
Interpretation:
The apparatus has to be redrawn if the temperature is decreased from
Concept introduction:
General Gas Law:
Combining Charles’s law and Boyle’s law we get the General gas law or combined gas law.
(c)
Answer to Problem 8.26UKC
The volume remains unchanged and the apparatus is redrawn as given below:
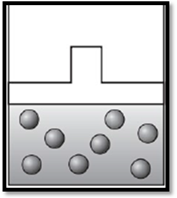
Explanation of Solution
Given data is that the temperature is decreased from
Combined gas law is proposed by combining the Boyle’s law and Charle’s law and is given by:
Therefore, the volume remains unchanged and the apparatus is redrawn as given below:
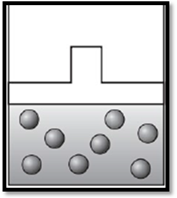
Want to see more full solutions like this?
Chapter 8 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
- Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5arrow_forwardShow a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forward
- Don't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forwardhow would you make this plot in excel?arrow_forwardwhat is the productarrow_forward
- Balance the following equation and list of coefficients in order from left to right. SF4+H2O+—-> H2SO3+HFarrow_forwardProblem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning- Basic Clinical Lab Competencies for Respiratory C...NursingISBN:9781285244662Author:WhitePublisher:Cengage





