
Concept explainers
(a)
Interpretation:
The apparatus has to be redrawn if the temperature is increased from
Concept introduction:
Charles’s law: States that volume is directly proportional to temperature when the gas is held at constant pressure and number of molecules.
(a)
Answer to Problem 8.26UKC
According to Charles’s law, if temperature increases at constant pressure, then volume also increases. Therefore, the apparatus is redrawn as given below:
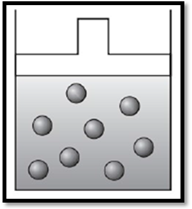
Explanation of Solution
Given data is that the temperature increases from
Charles’s law is the
From Charles’s law we know that
According to Charles’s law, if temperature increases at constant pressure, then volume also increases. Therefore, the apparatus is redrawn as given below:
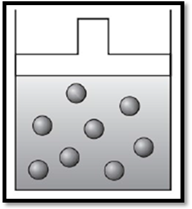
(b)
Interpretation:
The apparatus has to be redrawn if the pressure is increased from
Concept introduction:
Boyle’s law:
At fixed temperature, the volume of a fixed amount of gas is inversely proportional to the pressure exerted by the gas.
(b)
Answer to Problem 8.26UKC
According to Boyle’s law, if pressure increases at constant temperature, then volume decreases. Therefore, the apparatus is redrawn as given below:
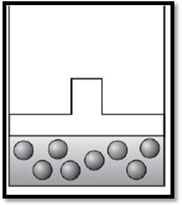
Explanation of Solution
Given data is that the pressure is increased from
Boyle’s law is the law which relates pressure and volume at a constant temperature and number of molecules.
From Boyle’s law:
According to Boyle’s law, if pressure increases at constant temperature, then volume decreases. Therefore, the apparatus is redrawn as given below:
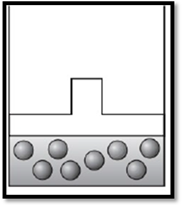
(c)
Interpretation:
The apparatus has to be redrawn if the temperature is decreased from
Concept introduction:
General Gas Law:
Combining Charles’s law and Boyle’s law we get the General gas law or combined gas law.
(c)
Answer to Problem 8.26UKC
The volume remains unchanged and the apparatus is redrawn as given below:
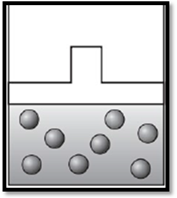
Explanation of Solution
Given data is that the temperature is decreased from
Combined gas law is proposed by combining the Boyle’s law and Charle’s law and is given by:
Therefore, the volume remains unchanged and the apparatus is redrawn as given below:
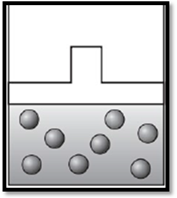
Want to see more full solutions like this?
Chapter 8 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- In a cell free preparation of beta-lactamase, penicillin is hydrolyzed in a D2O enriched assay. After one round of catalysis, where would you anticipate finding Deuterium? please help thank youarrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. question: the b-lactamase hydrolyzes the lactam-ring in antibiotics like penicillin. Describe the mechanism, of hydrolysis, insuring to include the involvement of S, D, and K in the reaction sequence. Please help!arrow_forwardThree of these amino acids participate in the proteolytic hydrolysis of polypeptides. Show the charge-relay network generated by the serine proteases and identify the nucleophilic species that initiates the hydrolysis. please help!arrow_forward
- You have isolated a protein and determined that the native molecular weight of the holoenzyme is 160 kD using size exclusion chromatography. Analysis of this protein using SDS-PAGE revealed 2 bands, one at 100 kD and one at 30 kD. 1. Describe the architecture of the polypeptide component of this enzyme. 2. The enzyme was found to be 0.829% NAD (by weight). What further can be said regarding the architecture? can you please help me with question number 2arrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Question: although S, K, and D are involved in the catalysis, the E in this hexapeptide does not participate in the hydrolysis of the b-lactam ring. Why is that?arrow_forwardTo map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. a) Using the experimental results described below deduce the primary sequence of the active site hexapeptide. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. please help!arrow_forward
- The beta-lactamase hydrolyzes the lactam-ring in penicillin. Describe the mechanism of hydrolysis, insuring to include the involvement of S, D, & K in the reaction sequence. Please helparrow_forwardTo map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Why doesn't D in this hexapeptide not participate in the hydrolysis of the beta-lactam ring even though S, K, and D are involved in the catalyst?arrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Using the experimental results described above derive the primary sequence of the active site hexapeptide. Please help!arrow_forward
- Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward+NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning- Basic Clinical Lab Competencies for Respiratory C...NursingISBN:9781285244662Author:WhitePublisher:Cengage





