
(a)
Interpretation:
The full set of possible quantum numbers for the outermost electron in
Concept introduction:
The electrons in the outermost occupied shell that determine the chemical properties of the elements are called the outermost electrons.
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
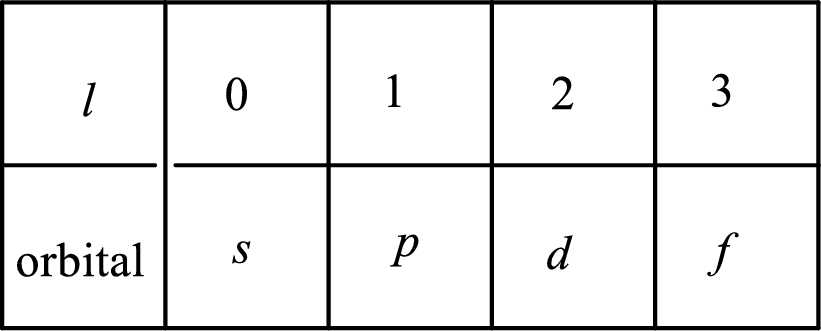
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(a)
Answer to Problem 8.21P
The possible quantum numbers for the outermost electron in
Explanation of Solution
The
Its outermost electron enters in the
The possible quantum numbers for the outermost electron in
(b)
Interpretation:
The full set of possible quantum numbers for the electron gained when an
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
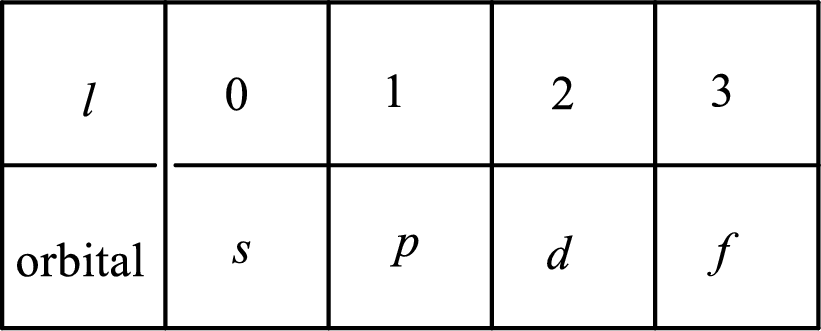
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(b)
Answer to Problem 8.21P
The possible quantum numbers for the electron gained when an
Explanation of Solution
The atomic number of sulfur is 16 so its electronic configuration is
The ion formation occurs as:
Its outermost electron enters in the
The value of the magnetic quantum number
The electron added to the
The possible quantum numbers for the electron gained when an
(c)
Interpretation:
The full set of possible quantum numbers for the electron lost when an
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
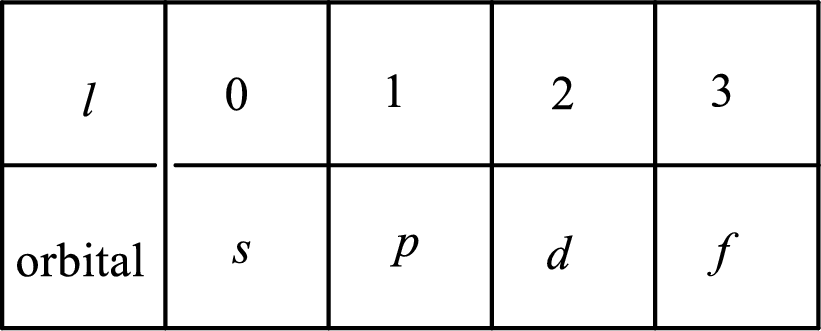
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(c)
Answer to Problem 8.21P
The possible quantum numbers for the electron lost when gained when an
Explanation of Solution
The atomic number of silver is 47 so its electronic configuration is
The ion formation occurs as:
The electron is lost from the
The possible quantum numbers for the electron lost when gained when an
(d)
Interpretation:
The full set of possible quantum numbers for the electron gained when an
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
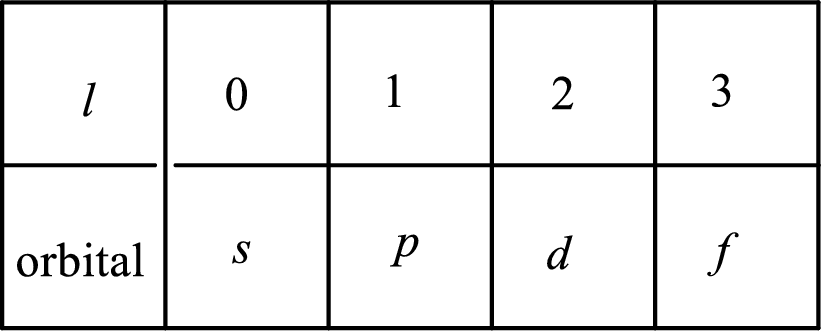
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(d)
Answer to Problem 8.21P
The quantum numbers for the electron gained when an
Explanation of Solution
The atomic number of fluorine is 9 so its electronic configuration is
The ion formation occurs as:
The electron is added to the
The value of the magnetic quantum number
The value of the spin quantum number
The quantum numbers for the electron gained when an
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry: The Molecular Nature of Matter and Change
- Which of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forwardWhich element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forwardWhich of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forward
- Which of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forwardConsider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forward
- Consider the redox reaction: 2 P4 + 8 OH− + 4 H2O → 4 PH3 + 4 HPO32− The element oxidized is ["", "", ""] , the element reduced is ["", "", ""] , one of the oxidizing agents is ["", "", ""] , and the reducing agent is ["", "", ""] .arrow_forwardWhat is the missing reactant in this organic reaction? OH H + R Δ CH3-CH2-CH-CH3 O CH3 CH3-CH2-C-O-CH-CH2-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answe box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C O2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cerarrow_forwardPredict the product of this organic reaction: CH3 NH2 Δ CH3-CH-CH3 + HO-C-CH2-N-CH3 P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. Xarrow_forward
- In the scope of the SCH4U course, please thoroughly go through the second questionarrow_forwardPlease help me solve these two problems. Thank you in advance.arrow_forwardNaming and drawing unsubstituted esters Write the systematic name of each organic molecule: Explanation structure Check name Х 2/5arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





