
Concept explainers
(a)
Interpretation:
The standard enthalpy of reaction has to be calculated using the values of bond dissociation enthalpies.
Concept Introduction:
The value of standard enthalpy change
Where,
(a)
Explanation of Solution
Given reaction (1):
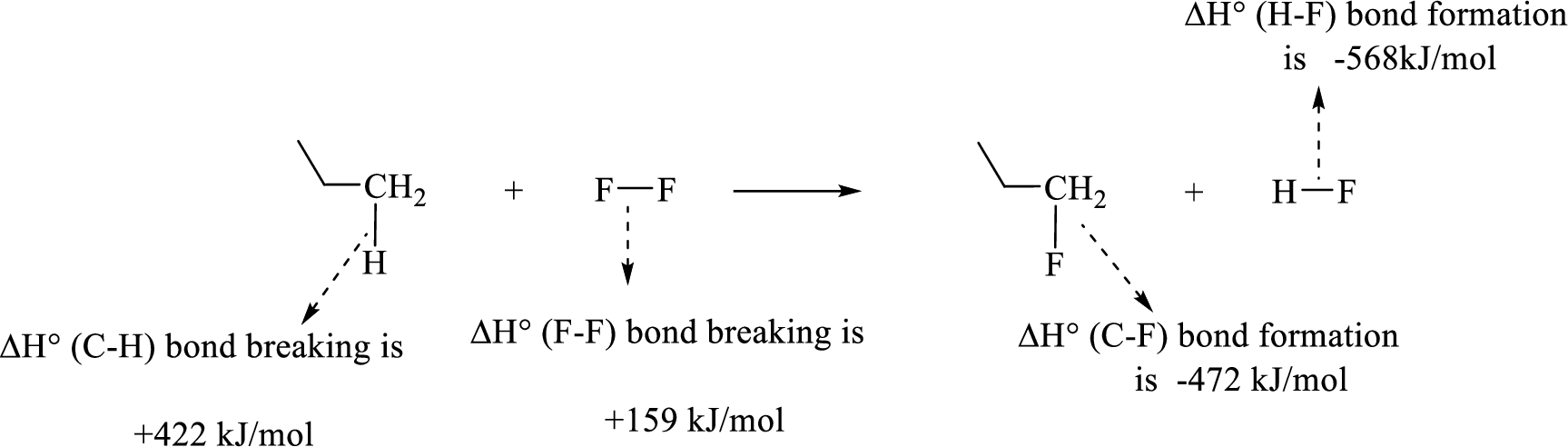
The reaction is given below:
Therefore, standard enthalpy of given reaction is
Given reaction (2):
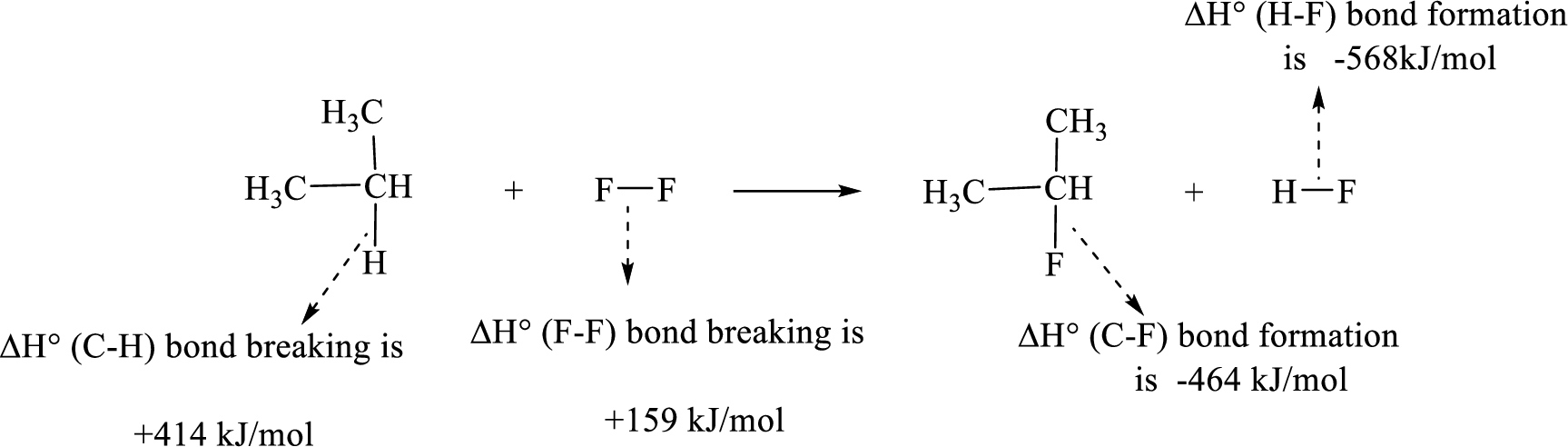
The reaction is given below:
Therefore, standard enthalpy of given reaction is
(b)
Interpretation:
A pair of chain propagation steps for each reaction has to be proposed; the value of
Concept Introduction:
Radical Bromination: A type of halogenation reaction in which the bromine atom gets bonded with the
Radical reaction of
Stability of Radicals: Radicals are highly unstable due to its unpaired valence electron of an atom.
The increasing order of radical stability is given below,
Benzylic > allylic > tertiary > secondary > primary > methyl
Radical chain reaction:
Initiation reaction: 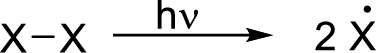
Chain propagation: 
Chain termination:
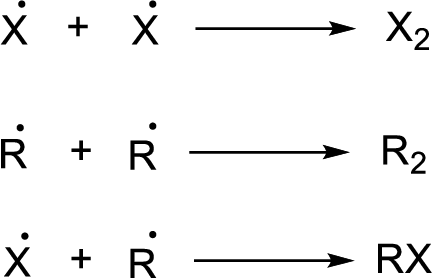
(b)
Explanation of Solution
Given reaction (1):
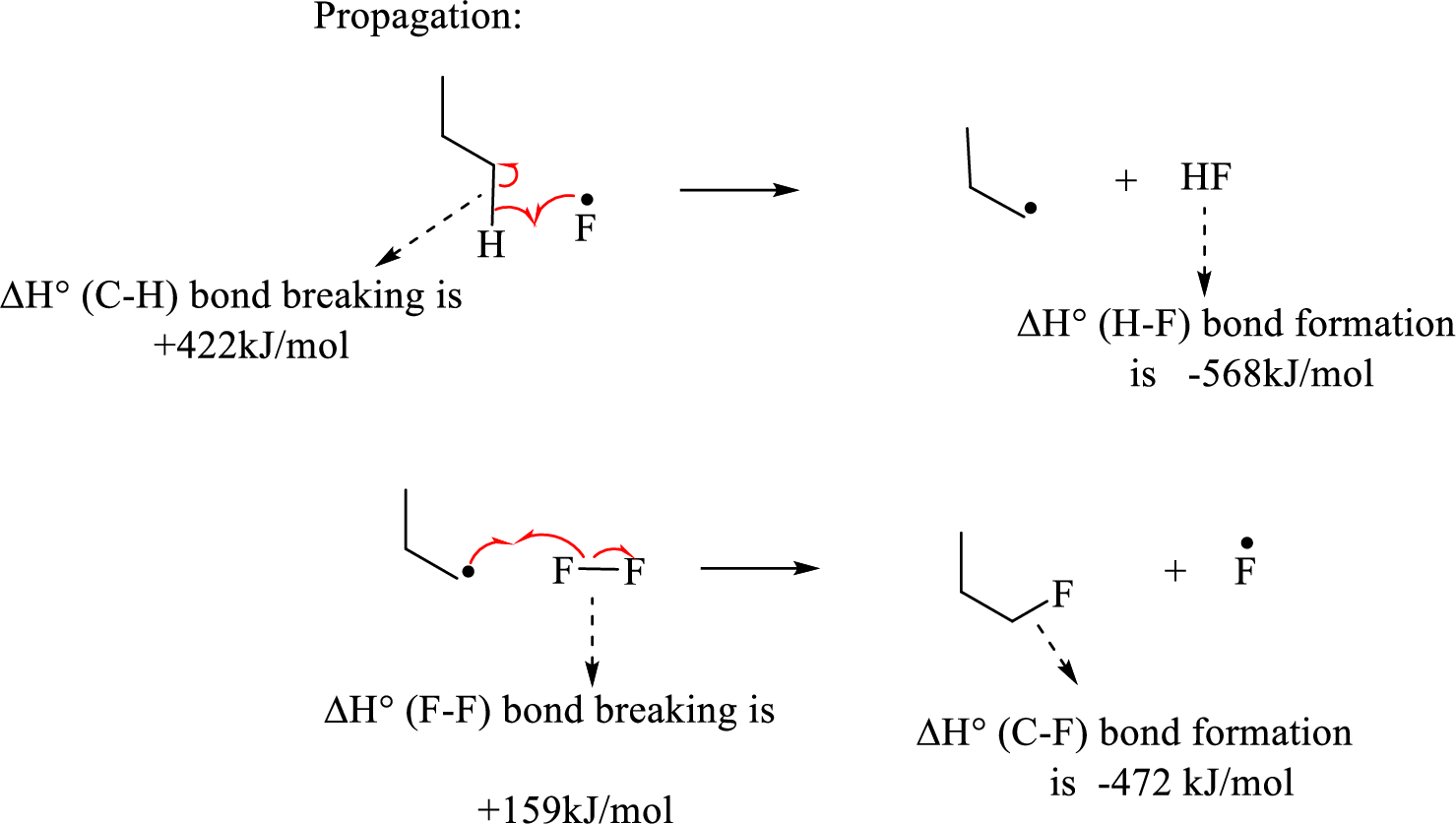
The propagation step involves extremely reactive fluorine radicals react towards the propane by abstracting the terminal hydrogen forming primary radical and
Hence, the pair of propagation steps is shown above.
Given reaction (2):
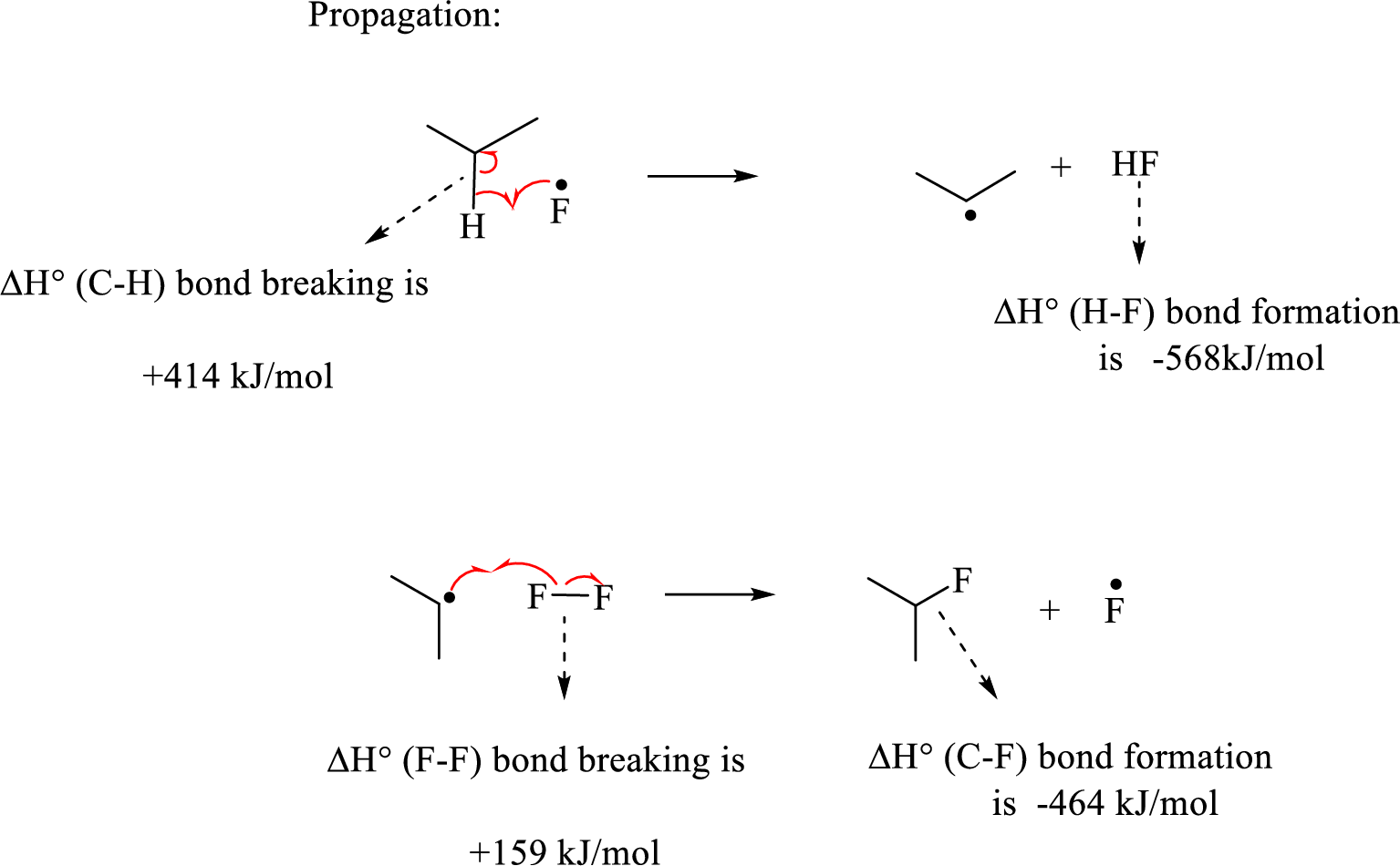
Initiation step involves the homolytic bond cleavage of fluorine molecule by irradiation of light or heat leads to the formation of two Fluorine radicals.
The propagation step involves extremely reactive fluorine radicals react towards the propane by abstracting the secondary hydrogen forming secondary radical and
Hence, the pair of propagation steps is shown above.
Therefore, for each reaction step the value of
(c)
Interpretation:
The regioselectivity of radical fluorination relative to that of radical chlorination and Bromination has to be predicted using Hammond’s postulate.
Concept Introduction:
Radical Bromination: A type of halogenation reaction in which the fluorine atom gets bonded with the alkane or alkyl substituents resulting to the product with added fluorine atom via radical mechanism.
Radical reaction of
Stability of Radicals: Radicals are highly unstable due to its unpaired valence electron of an atom.
The increasing order of radical stability is given below,
Benzylic > allylic > tertiary > secondary > primary > methyl
Radical chain reaction:
Initiation reaction: 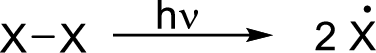
Chain propagation: 
Chain termination:
(c)
Explanation of Solution
In each radical fluorination sequence, the hydrogen abstraction step is highly exothermic, thus the transition state is reached too early and the transition state more closely resembles the reactant; so the intermediate has very little radical character of product. Therefore, the relative stabilities of primary versus secondary radical is of little of importance in determination of product.
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry, Loose-leaf Version
- In Potassium mu-dihydroxydicobaltate (III) tetraoxalate K4[Co2(C2O4)4(OH)2], indicate whether the OH ligand type is bidentate.arrow_forwardImagine an electrochemical cell based on these two half reactions with electrolyte concentrations as given below: Oxidation: Pb(s) → Pb2+(aq, 0.10 M) + 2 e– Reduction: MnO4–(aq, 1.50 M) + 4 H+(aq, 2.0 M) + 3 e– → MnO2(s) + 2 H2O(l) Calculate Ecell (assuming temperature is standard 25 °C).arrow_forward: ☐ + Draw the Fischer projection of the most common naturally-occurring form of aspartate, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. ✓arrow_forward
- For a silver-silver chloride electrode, the following potentials are observed: E°cell = 0.222 V and E(saturated KCl) = 0.197 V Use this information to find the [Cl–] (technically it’s the activity of Cl– that’s relevant here, but we’ll just call it “concentration” for simplicity) in saturated KCl.arrow_forwardA concentration cell consists of two Sn/Sn2+ half-cells. The cell has a potential of 0.10 V at 25 °C. What is the ratio of [Sn2+] (i.e., [Sn2+left-half] / [Sn2+right-half])?arrow_forwardElectrochemical cell potentials can be used to determine equilibrium constants that would be otherwise difficult to determine because concentrations are small. What is Κ for the following balanced reaction if E˚ = +0.0218 V? 3 Zn(s) + 2 Cr3+(aq) → 3 Zn2+(aq) + Cr(s) E˚ = +0.0218 Varrow_forward
- Consider the following half-reactions: Hg2+(aq) + 2e– → Hg(l) E°red = +0.854 V Cu2+(aq) + 2e– → Cu(s)E°red = +0.337 V Ni2+(aq) + 2e– → Ni(s) E°red = -0.250 V Fe2+(aq) + 2e– → Fe(s) E°red = -0.440 V Zn2+(aq) + 2e– → Zn(s) E°red = -0.763 V What is the best oxidizing agent shown above (i.e., the substance that is most likely to be reduced)?arrow_forwardCalculate the equilibrium constant, K, for MnO2(s) + 4 H+(aq) + Zn(s) → Mn2+(aq) + 2 H2O(l) + Zn2+(aq)arrow_forwardIn the drawing area below, draw the condensed structures of formic acid and ethyl formate. You can draw the two molecules in any arrangement you like, so long as they don't touch. Click anywhere to draw the first atom of your structure. A C narrow_forward
- Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule Ο C=O common name (not the IUPAC name) H ☐ H3N CH₂OH 0- C=O H NH3 CH₂SH H3N ☐ ☐ X Garrow_forward(Part A) Provide structures of the FGI products and missing reagents (dashed box) 1 eq Na* H* H -H B1 B4 R1 H2 (gas) Lindlar's catalyst A1 Br2 MeOH H2 (gas) Lindlar's catalyst MeO. OMe C6H1402 B2 B3 A1 Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forwardClassify each of the amino acids below. Note for advanced students: none of these amino acids are found in normal proteins. X CH2 H3N-CH-COOH3N-CH-COO- H3N-CH-COO CH2 CH3-C-CH3 CH2 NH3 N NH (Choose one) ▼ (Choose one) S CH2 OH (Choose one) ▼ + H3N-CH-COO¯ CH2 H3N CH COO H3N-CH-COO CH2 오오 CH CH3 CH2 + O C CH3 O= O_ (Choose one) (Choose one) ▼ (Choose one) Garrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
