
Pearson eText Basic Chemistry -- Instant Access (Pearson+)
6th Edition
ISBN: 9780135765982
Author: Karen Timberlake, William Timberlake
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 60CP
In the following diagram, if red spheres are the element A, white spheres are the element B. and green spheres are the element C: (8.1, 8.2, 8.3)
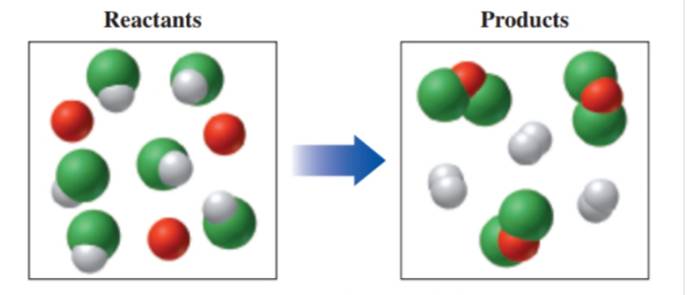
a. Write the formula for each of the reactants and products.
b. Write a balanced equation for the reaction.
c. Indicate the type of reaction as combination, decomposition, single replacement, double replacement. or combustion.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me to figure this out. I got 24 is that correct? Please step by step help.
The initial rates method can be used to
determine the rate law for a reaction.
using the data for the reaction below, what is
the rate law for reaction?
A+B-C
-
ALA]
At
(mot
Trial [A] (mol)
(MD
2
1
0.075
[B](
0.075
mo
LS
01350
2
0.075
0.090 0.1944
3
0.090 0.075
0.1350
Report value of k with two significant Figure
Compare trials 1 and 2 where [B] is
constant.
The rate law can be written as: rate
= k[A][B]".
rate2
0.090
= 9.
rate1
0.010
[A]m
6.0m
= 3m
[A] m
2.0m
Chapter 8 Solutions
Pearson eText Basic Chemistry -- Instant Access (Pearson+)
Ch. 8.1 - State the number of atoms of oxygen in the...Ch. 8.1 - State the number of atoms of oxygen in the...Ch. 8.1 - Prob. 3PPCh. 8.1 - Determine whether each of the following equations...Ch. 8.1 - All of the following are balanced equations. State...Ch. 8.1 - All of the following are balanced equations. State...Ch. 8.2 - Balance each of the following chemical...Ch. 8.2 - Balance each of the following chemical...Ch. 8.2 - Prob. 9PPCh. 8.2 - Prob. 10PP
Ch. 8.2 - Balance each of the following chemical...Ch. 8.2 - Prob. 12PPCh. 8.2 - Write a balanced equation using the correct...Ch. 8.2 - Write a balanced equation using the correct...Ch. 8.2 - Dinitrogen oxide, also known as laughing gas, is a...Ch. 8.2 - When ethanol C2H6O(aq) is consumed, it reacts with...Ch. 8.2 - In the body, the amino acid alanine C3H7NO2(aq)...Ch. 8.2 - Prob. 18PPCh. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Using Table 8.3, predict the products that would...Ch. 8.3 - Using Table 8.3, predict the products that would...Ch. 8.4 - Prob. 25PPCh. 8.4 - Identify each of the following as an oxidation or...Ch. 8.4 - Prob. 27PPCh. 8.4 - In each of the following, identify the reactant...Ch. 8.4 - In the mitochondria of human cells, energy is...Ch. 8.4 - Prob. 30PPCh. 8.4 - When linoleic acid, an unsaturated fatty acid,...Ch. 8.4 - Prob. 32PPCh. 8.4 - a. During cellular respiration, aqueous glucose...Ch. 8.4 - Aqueous fatty acids undergo reaction with oxygen...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - Prob. 39UTCCh. 8 - The chapter sections to review are shown in...Ch. 8 - Prob. 41UTCCh. 8 - If blue spheres represent nitrogen atoms, purple...Ch. 8 - Identify the type of reaction for each of the...Ch. 8 - Identify the type of reaction for each of the...Ch. 8 - Balance each of the following chemical equations,...Ch. 8 - Balance each of the following chemical equations,...Ch. 8 - Predict the products and write a balanced equation...Ch. 8 - Prob. 48APPCh. 8 - Write a balanced equation for each of the...Ch. 8 - Write a balanced equation for each of the...Ch. 8 - Prob. 51APPCh. 8 - Prob. 52APPCh. 8 - Prob. 53CPCh. 8 - Prob. 54CPCh. 8 - Prob. 55CPCh. 8 - Prob. 56CPCh. 8 - The following problems are related to the topics...Ch. 8 - Prob. 58CPCh. 8 - Prob. 59CPCh. 8 - In the following diagram, if red spheres are the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forwardCan you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forwardCan you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forward
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY