
Concept explainers
A diagram for an open-tube manometer is shown below.
If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations where a gas is contained in the flask, calculate the pressure in the flask in torr, atmospheres, and pascals.
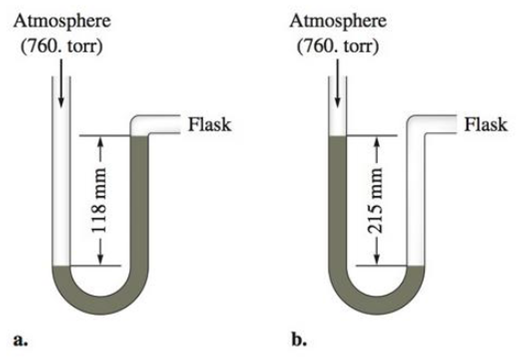
c. Calculate the pressures in the flask in parts a and b (in torr) if the atmospheric pressure is 635 torr.
(a)
Interpretation:
The pressure of the gases in given two situations of manometers (a) and (b) should be determined in units of torr, atm and pascals when the manometer shows a reading of 118mm and 215mm respectively. And also calculate the pressure of the gases in given two situations of manometers (a) and (b) If the atmospheric pressure is 635 torr.
Concept Introduction:
The manometer is a devise used measure the pressure of a gas. The pressure of gas is determined by the value of ‘h’ shown by the manometer. This ‘h’-value is added or subtracted with atmospheric pressure to determine the pressure of gas.
If the flask side mercury level is decreased after the filling of gas, then the ‘h’-value will be added to atmospheric pressure to get the pressure of gas.
If the flask side mercury level is increased after the filling of gas, then the ‘h’-value will be subtracted from the atmospheric pressure to get the pressure of gas.
The pressure equivalent of ‘h’ value is,
Pressure of a substance can be stated in various units. The units of pressure are interconvertible. The relations between units of pressure are,
- Since the unit mm Hg and the unit torr is used interchangeably.
- Conversion of 1 torr into atm is,
- The 1 mm Hg pressure in Pa unit is,
Answer to Problem 41E
The pressure of the given gas (figure-a) in units of torr, atm and pascal are,
642 torr, 0.8447 atm, 85593 Pa
Explanation of Solution
The given ‘h’ value for the gas in manometer is 118mm. The picture of manometer shows the flask side mercury level is increased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 642 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is 642mm Hg. So the pressure in atm unit is,
=
The calculated pressure is 642mm Hg. So the pressure in Pa unit is,
(b)
Interpretation:
The pressure of the gases in given two situations of manometers (a) and (b) should be determined in units of torr, atm and pascals when the manometer shows a reading of 118mm and 215mm respectively. And also calculate the pressure of the gases in given two situations of manometers (a) and (b) If the atmospheric pressure is 635 torr.
Concept Introduction:
The manometer is a devise used measure the pressure of a gas. The pressure of gas is determined by the value of ‘h’ shown by the manometer. This ‘h’-value is added or subtracted with atmospheric pressure to determine the pressure of gas.
If the flask side mercury level is decreased after the filling of gas, then the ‘h’-value will be added to atmospheric pressure to get the pressure of gas.
If the flask side mercury level is increased after the filling of gas, then the ‘h’-value will be subtracted from the atmospheric pressure to get the pressure of gas.
The pressure equivalent of ‘h’ value is,
Pressure of a substance can be stated in various units. The units of pressure are interconvertible. The relations between units of pressure are,
- Since the unit mm Hg and the unit torr is used interchangeably.
- Conversion of 1 torr into atm is,
- The 1 mm Hg pressure in Pa unit is,
Answer to Problem 41E
The pressure of the given gas (figure-b) in units of torr, atm and pascal are,
878 torr, 1.1552 atm, 117057 Pa
Explanation of Solution
The given ‘h’ value for the gas in manometer is 118mm. The picture of manometer shows the flask side mercury level is decreased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 878 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is 878mm Hg. So the pressure in atm unit is,
=
The calculated pressure is 878mm Hg. So the pressure in Pa unit is,
(c)
Interpretation:
The pressure of the gases in given two situations of manometers (a) and (b) should be determined in units of torr, atm and pascals when the manometer shows a reading of 118mm and 215mm respectively. And also calculate the pressure of the gases in given two situations of manometers (a) and (b) If the atmospheric pressure is 635 torr.
Concept Introduction:
The manometer is a devise used measure the pressure of a gas. The pressure of gas is determined by the value of ‘h’ shown by the manometer. This ‘h’-value is added or subtracted with atmospheric pressure to determine the pressure of gas.
If the flask side mercury level is decreased after the filling of gas, then the ‘h’-value will be added to atmospheric pressure to get the pressure of gas.
If the flask side mercury level is increased after the filling of gas, then the ‘h’-value will be subtracted from the atmospheric pressure to get the pressure of gas.
The pressure equivalent of ‘h’ value is,
Pressure of a substance can be stated in various units. The units of pressure are interconvertible. The relations between units of pressure are,
- Since the unit mm Hg and the unit torr is used interchangeably.
- Conversion of 1 torr into atm is,
- The 1 mm Hg pressure in Pa unit is,
Answer to Problem 41E
The pressure of the given gas (figure-a) in units of torr, atm and pascal when the atmospheric pressure is 635 torr are,
517 torr, 0.8141 atm, 82496 Pa
The pressure of the given gas (figure-b) in units of torr, atm and pascal when the atmospheric pressure is 635 torr are,
753 torr, 1.1858 atm, 120154 Pa
Explanation of Solution
The pressure of the gas in given situation of manometer (a) in units of torr, atm and pascals:
The given ‘h’ value for the gas in manometer (a) is 118mm. The picture of manometer shows the flask side mercury level is increased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 517 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is 517mm Hg. So the pressure in atm unit is,
=
The calculated pressure is 517mm Hg. So the pressure in Pa unit is,
The pressure of the gas in given situation of manometer (b) in units of torr, atm and pascals:
The given ‘h’ value for the gas in manometer is 118mm. The picture of manometer shows the flask side mercury level is decreased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 753 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is
=
The calculated pressure is
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry: An Atoms First Approach
- 6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward
- 9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forward
- x + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- Results Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





