
Concept explainers
Interpretation:
The structures of the major organic products formed in the reaction of
Concept introduction:
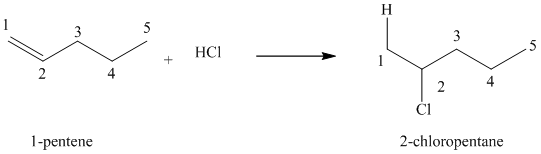
When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogens, and the halogen adds to the carbon that has fewer hydrogens. This rule is called Markovnikov’s rule.
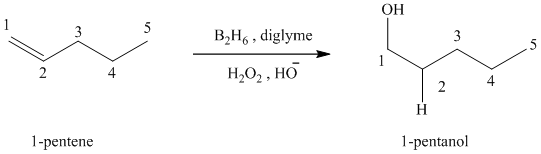
During hydroboration oxidation, hydrogen forms a bond with the carbon atom that has fewer hydrogens attached to it and the hydroxyl atom forms a bond with the carbon atom that has a greater number of hydrogens attached to it. This is a rule opposite to the Markovnikov’s addition.
Answer to Problem 28P
Solution:
Explanation of Solution
(a) Reaction of

The given alkene,
Hydrogen chloride gets added to the double bond of
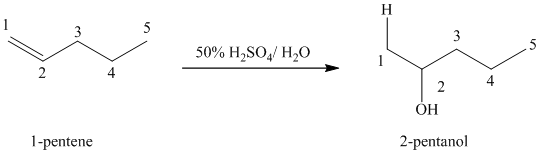
(b) Reaction of
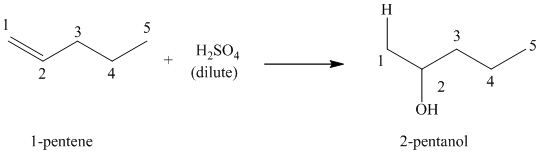
This reaction is an acid catalyzed electrophilic addition reaction of alkenes in which water molecule adds to the double bond in
A molecule of water adds to the double bond of
The addition mechanism for this reaction follows the Markovnikov’s rule. Therefore, the major organic product for the above acid-catalyzed electrophilic addition reaction is
(c) Reaction of
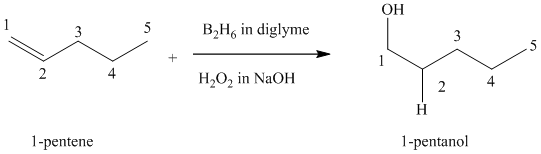
Hydroboration-oxidation leads to the overall hydration of an alkene. In hydroboration-oxidation,
The hydrogen atom in the water molecule adds to the carbon
In case of
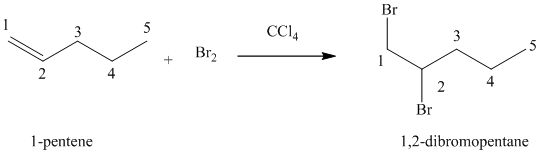
(d) Reaction of

Bromine reacts rapidly with alkenes by electrophilic addition. The products are called vicinal dibromides, meaning that the bromine atoms get attached to adjacent double bonded carbon atoms. It is carried out in suitable solvents like
A molecule of bromine adds across the double bond in
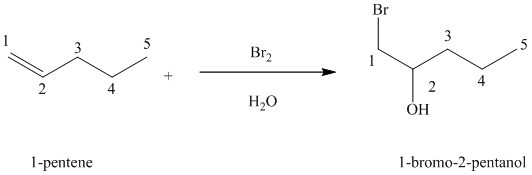
(e) Reaction of
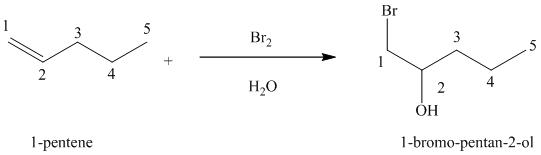
Chlorine and bromine react with alkenes in aqueous solution to give the corresponding vicinal halohydrins – compounds that add a halogen and hydroxyl group on adjacent carbon atoms in the alkene. The halogen atom forms a bond with that carbon atom in alkene, which has a greater number of hydrogen atoms, while the hydroxyl group bonds to that carbon atom in alkene, which has a fewer number of hydrogen atoms.
In the reaction of
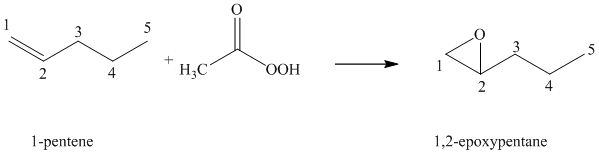
(f) Reaction of
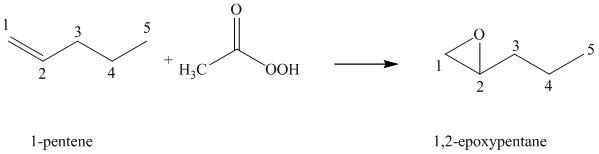
Peroxyacid transfers oxygen to the double bond of alkene to yield epoxides, which is a three-membered oxygen-containing ring.
When
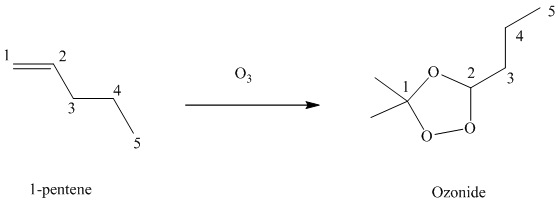
(g) Reaction of
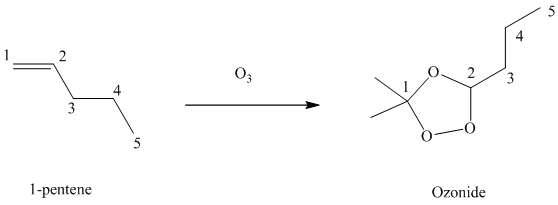
Ozone is a powerful electrophile and reacts with alkenes to cleave the double bond between two oxygen atoms in the molecule, forming an ozonide.
When
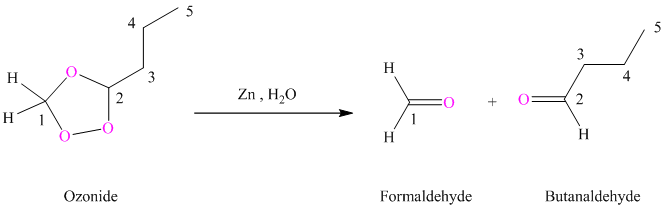
(h) Product of part (g) treated with zinc in water
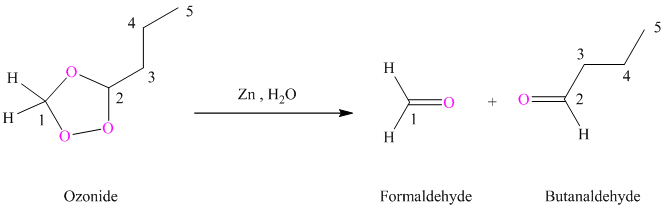
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water giving carbonyl compounds. Depending upon the structure of the starting alkene, various carbonyl compounds such as formaldehyde, aldehydes, or ketones are formed.
When corresponding ozonide of
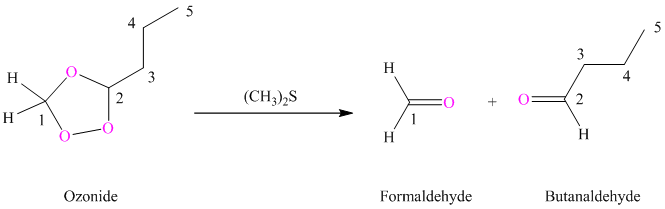
(i) Product of part (g) is treated with dimethyl sulfide.
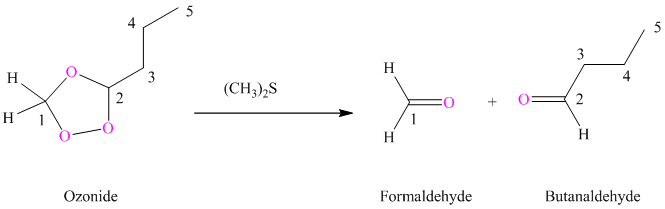
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water, giving carbonyl compounds. Depending upon the structure of the starting alkene, various carbonyl compounds such as formaldehyde, aldehydes, or ketones are formed.
When corresponding ozonide of
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





