
Concept explainers
Strike-anywhere matches contain a layer of
KCIO3 contains the
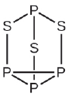
(a) Write Lewis structures for
(b) Describe the geometry about the P atoms, the S atom, and the Cl atom in these species.
(c) Assign a hybridization to the P atoms, the S atom, and the Cl atom in these species.
(d) Determine the oxidation states and formal charge of the atoms in
Trending nowThis is a popular solution!

Chapter 8 Solutions
Chemistry (OER)
Additional Science Textbook Solutions
Biological Science (6th Edition)
Fundamentals of Anatomy & Physiology (11th Edition)
Introductory Chemistry (6th Edition)
Microbiology: An Introduction
Biology: Life on Earth (11th Edition)
Applications and Investigations in Earth Science (9th Edition)
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardUsing what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forward
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardIndicate whether the copper(II) acetate dimer, in its dihydrated form with the formula [(CH3COO)2Cu]2·2H2O, is a metal cluster, a cage compound, or neither.arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


