
Concept explainers
a)
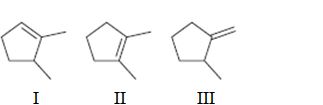
Interpretation:
To rank the double bond in the compounds given in terms of increasing stability.
Concept introduction:
The stability of a double bond can be determined by the number of hydrogens on adjacent carbons attached to both carbons in the double bond. (H’s on the α carbon). More the number of such hydrogens, the more hyperconjugation occur and more stable the
b)
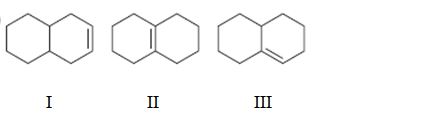
Interpretation:
To rank the double bond in the compounds given in terms of increasing stability.
Concept introduction:
The stability of a double bond can be determined by the number of hydrogens on adjacent carbons attached to both carbons in the double bond.(H’s on the α carbon). More the number of such hydrogens, the more hyperconjugation occur and more stable the alkene.
c)
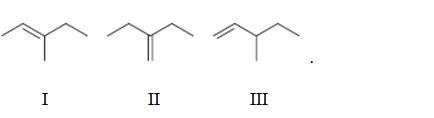
Interpretation:
To rank the double bond in the compounds given in terms of increasing stability.
Concept introduction:
The stability of a double bond can be determined by the number of hydrogens on adjacent carbons attached to both carbons in the double bond.(H’s on the α carbon). More the number of such hydrogens, the more hyperconjugation occur and more stable the alkene.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
- 11) The Ksp expression for copper (II) sulfate is: a. [Cu2+][SO4²¯] b. [Cu²+]² [SO4²]² c. [Cu²+]²[SO4²] d. [CuSO4] 12) Which of the following is true about a chemical system in equilibrium? a. All chemical reactions have stopped b. The concentration of reactants is equal to the concertation of products c. The forward and reverse reaction rates become equal d. The system will remain at equilibrium regardless of any external factorsarrow_forward21) Explain the difference between the rate of a reaction and the extent of a reaction. Why are both of these concepts important, if you are a chemical engineer that is trying to develop a process to produce a large volume of a specific type of chemical compound?arrow_forwardPls help.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


