
Concept explainers
Interpretation:
The given compound has to be named.
Concept Introduction:
All the molecules have their unique names. These names must be known in order to communicate. But remembering all the chemical names is impossible as there are many numbers of molecules. To avoid this, the
| Stereoisomerism | Substituents | Parent | Unsaturation | Functional Group |
Stereoisomerism indicate if the considered molecule has any stereocenters are present (R,S) and if double bond is present are cis/trans. In order to name a double bond as cis/trans, the important condition is that, an identical group has to be present on either side of the double bond. If the identical groups are present on the same side of double bond, then it is known as cis. If the identical groups are present on the opposite side of the double bond then it is known as trans.
The groups that are connected to the main carbon chain are known as substituents. The substituents are identified after the carbon chain is identified and the functional group present in the given compound also identified. The substituents are named by adding “yl” to the end of the name which indicate that it is a substituent is an alkyl. The
The longest carbon chain is known as the parent. Parent carbon chain is the lengthiest carbon chain in the molecule that must include the functional group that is present in the compound. The longest carbon chain is identified and the parent name is given by the number of carbon atoms that is present in it. It must be remembered that even though functional group is not present in parent carbon chain, the double bond, triple bond if present has to be included. Numbering becomes a part of all the parts in IUPAC name. After identifying the parent chain, the numbering is done. If functional group is present in the given compound, the numbering is given in such a way that the functional group gets the least number. Then the double bond, triple bond.
| Number of carbon atoms in chain | Parent |
| 1 | Meth |
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
| 6 | Hex |
Unsaturation indicates that if any triple or double bonds are present in the molecule. If a compound contains a double bond it is named as “-en-” and if a triple bond is present “-yn-” is used. If a compound contains two double bonds, then it is named as “-dien-”. If three double bonds are present in the given compound, then it is named as “-trien-”. This same rule applies for the compound that contains multiple triple bonds also.
Functional group is the one after which the considered compound is being named.
| Functional Group | Class of compound | Suffix |
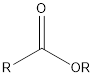 | Ester | -oate |
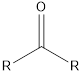 | Ketone | -one |
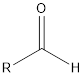 | Aldehyde | -al |
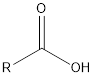 | -oic acid | |
 | Alcohol | -ol |
 | -amine |
For naming a given compound, the reverse order has to be followed. First the functional group is considered followed by unsaturation, parent chain, substituents, and then the stereoisomerism.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry As a Second Language: First Semester Topics
- In the image, the light blue sphere represents a mole of hydrogen atoms, the purple or teal spheres represent a mole of a conjugate base. A light blue sphere by itself is H+. Assuming there is 2.00 L of solution, answer the following: The Ka of the left & right solution is? The pH of the left & right solution is? The acid on the left & right is what kind of acid?arrow_forwardNonearrow_forwardNonearrow_forward
- Nonearrow_forwardWhat spectral features allow you to differentiate the product from the starting material? Use four separate paragraphs for each set of comparisons. You should have one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of functional group changes.arrow_forwardQuestion 6 What is the major product of the following Diels-Alder reaction? ? Aldy by day of A. H о B. C. D. E. OB OD Oc OE OAarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





