
Concept explainers
Interpretation:
The configuration of stereocenter has to be determined and the enantiomer has to be drawn for the given compound.
Concept Introduction:
If a molecule contains a carbon atom that is bonded to four different groups, then that carbon is said to be chiral carbon and the carbon center responsible for this chirality is known as stereocenter. Stereocenter are located in a compound by looking into the atoms or groups that is attached to the carbon atoms. An example of a compound having chiral center is shown below,
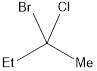
The stereocenter can have either R or S configuration. This can be identified by numbering the groups from 1 to 4 that is attached to the stereocenter. If the stereocenter has the same type of atoms attached to it, then the next atoms are considered. The way in which the numbering was given is based only on the
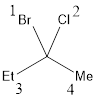
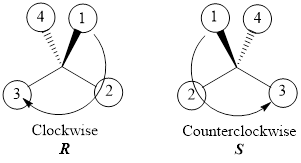
If the numbering goes clockwise, then the configuration will be “R” and if the numbering of the groups goes counterclockwise, then the configuration will be “S”. This can be represented as shown below,
The first and foremost condition is that the group with the least priority has to point away from us. If the group with least priority is not pointing away from us, then the group has to be switched in a way that the least priority group points away. By doing this the configuration of the stereocenter changes. After the switching is done and the group with least priority is pointing away from us, another switching has to be done with other groups so that the original configuration of the stereocenter is obtained.
Fischer projections are very much useful for drawing the molecules that contain more stereocenters. Fischer projections does not use wedge and dash bonds. Instead vertical and horizontal lines are used. Horizontal lines means they are pointing towards us and vertical lines means they are pointing away from us. Fischer projection can also be used for the compounds that contain only one stereocenter.
Enantiomers are the two compounds that are nonsuperimposable mirror images of each other. Enantiomers always come in pairs and they are mirror images of each other. If a compound is given with a chiral bond, the enantiomer of the same compound can be drawn by converting the stereobond alone. This means that all the dash bond has to be changed to wedge bond and wedge bond has to be changed into dash bond. Another method of drawing the enantiomer of the given compound that does not contain any wedge or dash bond is that to keep an imaginary mirror on the side of compound and draw the reflection that is got in the mirror.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry As a Second Language: First Semester Topics
- utron eutro cle TH tro (Na (b) Atoms are said to be electrically neutral. Explain. (c) Distinguish between the following: (i) Atomic number and mass number. (ii) Mass number and relative atomic mass. 2. An isotope Q, has 18 neutrons a mass number of 34. (a) (i) Draw the atomic structure of Q. (ii) Write its electron arrangement (b) To which period and group does Q belong? Explain your answer. (c) How does Q form its ion? Explain. 3. (a) Determine the relative atomic mass of the following elements = compositions occur in the proportions given. (i) Neon 20 21 22. Ne (90.92%), 10Ne (0.26%), and 10Ne (8.82%) (ii) Argon 36 38 40 18 Ar (0.34%), 18 Ar (0.06%) and 18 Ar (99.6%)arrow_forwardIn the normal hydrogen electrode, the balance potential difference in the interface is this, the maximum potential is 5 mV. Explain briefly.arrow_forwardThe electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The overvoltage n will be 0.005 - (-0.118) = 0.123 V. Is it correct?arrow_forward
- In the electrode Pt, H2(1 atm) | H+(a=1), if the electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The current voltage will be 0.005 - (-0.118) = 0.123 V ¿Correcto?arrow_forwardIn the electrode Pt, H2(1 atm) | H+(a=1) at 298K is 0.79 mA cm-2. If the balance potential of the electrode is -0.118 V and the potential difference of the interface is +5 mV. Determine its potential.arrow_forwardIn one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.arrow_forward
- In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forwardIf the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forward
- Topic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





