
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
4th Edition
ISBN: 9781119761068
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.3, Problem 7.11P
Predict the major product in each of the following cases:
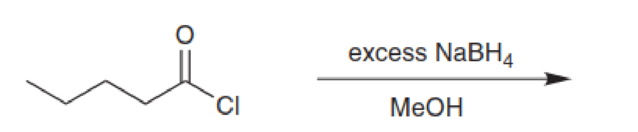
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
+ HCl →?
Draw the molecule on the canvas by choosing buttons from the Tools (for bonas), Atoms
and Advanced Template toolbars. The single bond is active by default.
+
M
C
+
H± 2D
EXP. CONT. K
?
L
1
H₁₂C
[1]
A
HCN O
S
CH3
CH 3
CI
Br
HC
H₂
CH
CH
CH3
-
P
F
SH
Chapter 7 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...
Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.5 - Prob. 7.23PCh. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - Prob. 7.33PCh. 7.5 - Prob. 7.34PCh. 7.5 - Prob. 7.35PCh. 7.5 - Prob. 7.36PCh. 7.6 - Based on everything we have just seen, propose a...Ch. 7.6 - Prob. 7.39PCh. 7.6 - Prob. 7.40PCh. 7.6 - Prob. 7.41PCh. 7.6 - Propose a mechanism for the following reaction:Ch. 7.6 - Prob. 7.44PCh. 7.6 - Prob. 7.45PCh. 7.6 - Prob. 7.46PCh. 7.7 - Prob. 7.48PCh. 7.7 - Prob. 7.49PCh. 7.7 - Prob. 7.50PCh. 7.7 - Prob. 7.51PCh. 7.7 - Prob. 7.52PCh. 7.7 - Prob. 7.53PCh. 7.7 - Prob. 7.55PCh. 7.7 - Prob. 7.56PCh. 7.7 - Prob. 7.57PCh. 7.7 - Prob. 7.58PCh. 7.7 - Prob. 7.59PCh. 7.7 - Prob. 7.60PCh. 7.7 - Prob. 7.61PCh. 7.7 - Prob. 7.62PCh. 7.7 - Prob. 7.63PCh. 7.7 - Prob. 7.64PCh. 7.7 - Prob. 7.65P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
7. Which bones form via intramembranous ossification?
a. Irregular bones
b. Certain flat bones
c. Long bones
d....
Human Anatomy & Physiology (2nd Edition)
16. ATP is synthesized from ADP, Pi, and a proton on the matrix side of the inner mitochondrial membrane. We wi...
Biochemistry: Concepts and Connections (2nd Edition)
48. Are these data sets on chemical changes consistent with the law of conservation of mass?
a. A 12.8-g sample...
Introductory Chemistry (6th Edition)
51. I A tennis player hits a ball 2.0 m above the ground. The ball leaves his racquet with a speed of 20.0 m/s ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- SH 0arrow_forward2. Please consider the two all 'cis' isomers of trimethylcyclohexane drawn below. Draw the two chair conformers of each stereoisomer below (1 and 2) and calculate their torsional interaction energies in order to identify the lower energy conformer for each stereoisomer. Based on your calculations, state which of the two stereoisomers 1 and 2 is less stable and which is more stable. [1,3-diaxial CH3 CH3 = 3.7kcal/mol; 1,3-diaxial CH3 H = 0.88kcal/mol; cis-1,2 (axial:equatorial) CH3 CH3 = 0.88kcal/mol; trans-1,2-diequatorial CH3 CH3 = 0.88kcal/mol) all-cis-1,2,3- 1 all-cis-1,2,4- 2arrow_forwardNonearrow_forward
- What is the mechanism by which the 1,4 product is created? Please draw it by hand with arrows and stuff.arrow_forwardWhat is the relationship between A and B? H3C A Br Cl H3C B Br relationship (check all that apply) O same molecule O enantiomer O diastereomer structural isomer O stereoisomer isomer O need more information to decide O same molecule ☐ enantiomer Br Br Br CH3 Br CI CH3 O diastereomer ☐ structural isomer ☐ stereoisomer isomer O need more information to decide O same molecule O enantiomer Odiastereomer structural isomer O stereoisomer ☐ isomer O need more information to decidearrow_forwardb. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 4arrow_forward
- c. Serricornin, the female-produced sex pheromone of the cigarette beetle, has the following structure. OH What is the maximum number of possible stereoisomers? Is this structure a meso compound? d. Please consider the natural product alkaloids shown below. Are these two structures enantiomers, diastereomers or conformers? H HO H H HN HO HN R R с R=H cinchonidine R=ET cinchonine Harrow_forwardNail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m. Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.arrow_forwardPlease help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship. I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?arrow_forward
- Describe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.arrow_forwardDon't used hand raitingarrow_forwardIt is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License