
Concept explainers
a)
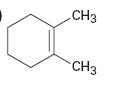
Interpretation:
The IUPAC name of the compound shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane – the cycloalkene is named with the suffix –ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To find:
The IUPAC name of the compound shown.
b)
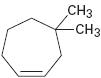
Interpretation:
The IUPAC name of the compound shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane – the cycloalkene is named with the suffix –ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To find:
The IUPAC name of the compound shown.
c)
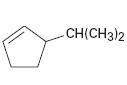
Interpretation:
The IUPAC name of the compound shown is to be given.
Concept introduction:
The maximum number of carbons in the ring is counted. Based on the name of the parent cycloalkane – the cycloalkene is named with the suffix –ene. The cycloalkane is numbered such that the double bond is in between C1 & C2 and the first substituent has the lowest number possible. Usually the position of a double bond is not shown in the name because it is always between C1 & C2. In dienes and trienes, however the position of double bonds is shown.
To find:
The IUPAC name of the compound shown.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- If CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forwardIs it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forwardStarting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forward
- Indicate the differences between the spectra of 1-methyl-benzimidazole and benzimidazole.arrow_forwardProvide reasons as to why appropriate sampling is important in relation to food?arrow_forwardWhat is the significance of selecting a "representative" sample for chemical analysis, and how does this practice ensure accurate and reliable results with respect to chemical analyses?arrow_forward
- Identify and provide an explanation of the differences between homogeneous and heterogeneous sampling in the context of sampling methods.arrow_forwardГ C-RSA CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.0 b.092 0.797 1.088 1.813 C-RSA CHROMATOPAC CH=1 Report No. =13 ** CALCULATION REPORT ** DATA=1: @CHRM1.000 11/03/05 08:09:52 CH PKNO TIME 1 2 0.797 3 1.088 4 1.813 AREA 1508566 4625442 2180060 HEIGHT 207739 701206 V 287554 V MK IDNO CONC NAME 18.1447 55.6339 26.2213 TOTAL 8314067 1196500 100 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0. 0 087 337. 0.841 1.150 C-R8A CHROMATOPAC CH=1 Report No. =14 DATA=1: @CHRM1.000 11/03/05 08:12:40 ** CALCULATION REPORT ** CH PKNO TIME AREA 1 3 0.841 1099933 41.15 4039778 HEIGHT MK IDNO 170372 649997¯¯¯ CONC NAME 21.4007 78.5993 TOTAL 5139711 820369 100 3 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.100 0:652 5.856 3 1.165 C-RSA CHROMATOPAC CH-1 Report No. =15 DATA=1: @CHRM1.000 11/03/05 08:15:26 ** CALCULATION REPORT ** CH PKNO TIME AREA HEIGHT MK IDNO CONC NAME 1 3 3 0.856 4 1.165 TOTAL 1253386 4838738 175481 708024 V 20.5739 79.4261 6092124…arrow_forwardDraw the product of the reaction shown below. Ignore small byproducts that would evaporate please.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


