
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
6th Edition
ISBN: 9781260325294
Author: SMITH
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.3, Problem 4P
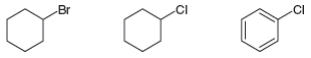
An
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
None
None
None
Chapter 7 Solutions
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
Ch. 7.1 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7.2 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7.2 - Prob. 3PCh. 7.3 - An sp3 hybridized CCl bond is more polar than an...Ch. 7.4 - Prob. 5PCh. 7.6 - Prob. 6PCh. 7.7 - Prob. 10PCh. 7.8 - Prob. 15PCh. 7.8 - Prob. 16PCh. 7.8 - Prob. 17P
Ch. 7.11 - Prob. 18PCh. 7.11 - Prob. 19PCh. 7.11 - Draw the product of each SN2 reaction and indicate...Ch. 7.11 - Prob. 21PCh. 7.11 - Prob. 22PCh. 7.12 - What happens to the rate of an SN1 reaction under...Ch. 7.12 - Draw the products of each SN1 reaction and...Ch. 7.13 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7.15 - Problem 7.30 For each alkyl halide and...Ch. 7.15 - Prob. 30PCh. 7.15 - Prob. 31PCh. 7.15 - Prob. 32PCh. 7.15 - Prob. 33PCh. 7.15 - Prob. 34PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 62PCh. 7 - Prob. 63PCh. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 69PCh. 7 - Prob. 70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 72PCh. 7 - Prob. 78P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forwardChoose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forward
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forwardNonearrow_forward
- Show work. don't give Ai generated solutionarrow_forwardPart II. count the expected number of signals in the 1H-NMR spectrum of these compounds HO 0 одев * Cl -cl "D"arrow_forwardPart I. Create a splitting tree diagram to predict the multiplet pattern of proton Hb in the compound below: 3 (Assume that "Jab >>> ³JbC) Ha Hb He он Ha NH2 Ha HCarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY