
Concept explainers
(a)
Interpretation:
The possible product for the given reaction should be drawn.
Concept Introduction:
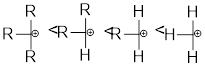
Leaving group: It is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Nucleophile: Donates pair of electrons to positively charged substrate resulting in the formation of
(b)
Interpretation:
The possible product for the given reaction should be drawn.
Concept Introduction:
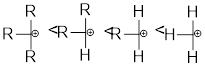
Leaving group: It is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Nucleophile: Donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
(c)
Interpretation:
The possible product for the given reaction should be drawn.
Concept Introduction:
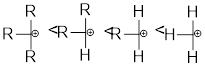
Leaving group: It is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Nucleophile: Donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
(d)
Interpretation:
The possible product for the given reaction should be drawn.
Concept Introduction:
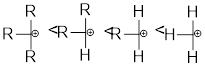
Leaving group: It is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Nucleophile: Donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
Trending nowThis is a popular solution!

Chapter 7 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
- Part 1. Draw monomer units of the following products and draw their reaction mechanism 1) Bakelite like polymer Using: Resorcinol + NaOH + Formalin 2) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerol 3) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardUsing the table of Reactants and Products provided provide the correct letter that corresponds with the Carboxylic acid that is formed in the reaction below. 6 M NaOH Acid-workup WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES A) Pool of Reagents for Part B CI B) OH C) E) CI J) racemic F) K) OH N) OH P) G) OH D) HO H) L) M) HO Q) R) CI Aarrow_forwardIn the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forward
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





